Mechanisms of GII.4 norovirus persistence in human populations
- PMID: 18271619
- PMCID: PMC2235898
- DOI: 10.1371/journal.pmed.0050031
Mechanisms of GII.4 norovirus persistence in human populations
Abstract
Background: Noroviruses are the leading cause of viral acute gastroenteritis in humans, noted for causing epidemic outbreaks in communities, the military, cruise ships, hospitals, and assisted living communities. The evolutionary mechanisms governing the persistence and emergence of new norovirus strains in human populations are unknown. Primarily organized by sequence homology into two major human genogroups defined by multiple genoclusters, the majority of norovirus outbreaks are caused by viruses from the GII.4 genocluster, which was first recognized as the major epidemic strain in the mid-1990s. Previous studies by our laboratory and others indicate that some noroviruses readily infect individuals who carry a gene encoding a functional alpha-1,2-fucosyltransferase (FUT2) and are designated "secretor-positive" to indicate that they express ABH histo-blood group antigens (HBGAs), a highly heterogeneous group of related carbohydrates on mucosal surfaces. Individuals with defects in the FUT2 gene are termed secretor-negative, do not express the appropriate HBGA necessary for docking, and are resistant to Norwalk infection. These data argue that FUT2 and other genes encoding enzymes that regulate processing of the HBGA carbohydrates function as susceptibility alleles. However, secretor-negative individuals can be infected with other norovirus strains, and reinfection with the GII.4 strains is common in human populations. In this article, we analyze molecular mechanisms governing GII.4 epidemiology, susceptibility, and persistence in human populations.
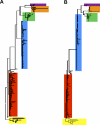
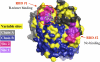
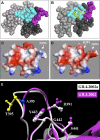
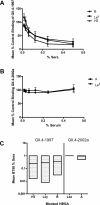
Methods and findings: Phylogenetic analyses of the GII.4 capsid sequences suggested an epochal evolution over the last 20 y with periods of stasis followed by rapid evolution of novel epidemic strains. The epidemic strains show a linear relationship in time, whereby serial replacements emerge from the previous cluster. Five major evolutionary clusters were identified, and representative ORF2 capsid genes for each cluster were expressed as virus-like particles (VLPs). Using salivary and carbohydrate-binding assays, we showed that GII.4 VLP-carbohydrate ligand binding patterns have changed over time and include carbohydrates regulated by the human FUT2 and FUT3 pathways, suggesting that strain sensitivity to human susceptibility alleles will vary. Variation in surface-exposed residues and in residues that surround the fucose ligand interaction domain suggests that antigenic drift may promote GII.4 persistence in human populations. Evidence supporting antigenic drift was obtained by measuring the antigenic relatedness of GII.4 VLPs using murine and human sera and demonstrating strain-specific serologic and carbohydrate-binding blockade responses. These data suggest that the GII.4 noroviruses persist by altering their HBGA carbohydrate-binding targets over time, which not only allows for escape from highly penetrant host susceptibility alleles, but simultaneously allows for immune-driven selection in the receptor-binding region to facilitate escape from protective herd immunity.
Conclusions: Our data suggest that the surface-exposed carbohydrate ligand binding domain in the norovirus capsid is under heavy immune selection and likely evolves by antigenic drift in the face of human herd immunity. Variation in the capsid carbohydrate-binding domain is tolerated because of the large repertoire of similar, yet distinct HBGA carbohydrate receptors available on mucosal surfaces that could interface with the remodeled architecture of the capsid ligand-binding pocket. The continuing evolution of new replacement strains suggests that, as with influenza viruses, vaccines could be targeted that protect against norovirus infections, and that continued epidemiologic surveillance and reformulations of norovirus vaccines will be essential in the control of future outbreaks.
Conflict of interest statement
Figures




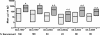

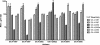

Comment in
-
The evolution of norovirus, the "gastric flu".PLoS Med. 2008 Feb;5(2):e42. doi: 10.1371/journal.pmed.0050042. PLoS Med. 2008. PMID: 18271623 Free PMC article. Review.
References
-
- Estes MK, Prasad BV, Atmar RL. Noroviruses everywhere: has something changed. Curr Opin Infect Dis. 2006;19:467–474. - PubMed
-
- Koopmans M, Vinje J, de Wit M, Leenen I, van der Poel W, et al. Molecular epidemiology of human enteric caliciviruses in The Netherlands. J Infect Dis. 2000;181(Suppl 2):S262–269. - PubMed
-
- [No authors listed] Norovirus activity—United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2007;56:842–846. - PubMed
-
- Johnston CP, Qiu H, Ticehurst JR, Dickson C, Rosenbaum P, et al. Outbreak management and implications of a nosocomial norovirus outbreak. Clin Infect Dis. 2007;45:534–540. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

