Natural variation in four human collagen genes across an ethnically diverse population
- PMID: 18272325
- PMCID: PMC2737816
- DOI: 10.1016/j.ygeno.2007.12.008
Natural variation in four human collagen genes across an ethnically diverse population
Abstract
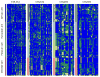
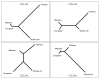
Collagens are members of one of the most important families of structural proteins in higher organisms. There are 28 types of collagens encoded by 43 genes in humans that fall into several different functional protein classes. Mutations in the major fibrillar collagen genes lead to osteogenesis imperfecta (COL1A1 and COL1A2 encoding the chains of Type I collagen), chondrodysplasias (COL2A1 encoding the chains of Type II collagen), and vascular Ehlers-Danlos syndrome (COL3A1 encoding the chains of Type III collagen). Over the past 2 decades, mutations in these collagen genes have been catalogued, in hopes of understanding the molecular etiology of diseases caused by these mutations, characterizing the genotype-phenotype relationships, and developing robust models predicting the molecular and clinical outcomes. To achieve these goals better, it is necessary to understand the natural patterns of variation in collagen genes in human populations. We screened exons, flanking intronic regions, and conserved noncoding regions for variations in COL1A1, COL1A2, COL2A1, and COL3A1 in 48 individuals from each of four ethnically diverse populations. We identified 459 single-nucleotide polymorphisms (SNPs), more than half of which were novel and not found in public databases. Of the 52 SNPs found in coding regions, 15 caused amino acid substitutions while 37 did not. Although the four collagens have similar gene and protein structures, they have different molecular evolutionary characteristics. For example, COL1A1 appears to have been under substantially stronger negative selection than the rest. Phylogenetic analysis also suggests that the four genes have very different evolutionary histories among the different ethnic groups. Our observations suggest that the study of collagen mutations and their relationships with disease phenotypes should be performed in the context of the genetic background of the subjects.
Figures
References
-
- Chu ML, Prockop DJ. Collagen: Gene Structure. In: Royce PM, Steinmann B, editors. Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects. Wiley-Liss; New York, NY: 2002. pp. 223–248.
-
- Kielty CM, Grant ME. The Collagen Family: Structure, Assembly, and Organization in the Extracellular Matrix. In: Royce PM, Steinmann B, editors. Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects. Wiley-Liss; New York, NY: 2002. pp. 159–221.
-
- Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, Hyland JC, Korkko J, Prockop DJ, De Paepe A, Coucke P, Symoens S, Glorieux FH, Roughley PJ, Lund AM, Kuurila-Svahn K, Hartikka H, Cohn DH, Krakow D, Mottes M, Schwarze U, Chen D, Yang K, Kuslich C, Troendle J, Dalgleish R, Byers PH. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28:209–21. - PMC - PubMed
-
- Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342:673–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

