A chemical method for fast and sensitive detection of DNA synthesis in vivo
- PMID: 18272492
- PMCID: PMC2268151
- DOI: 10.1073/pnas.0712168105
A chemical method for fast and sensitive detection of DNA synthesis in vivo
Abstract
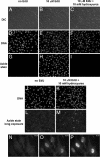
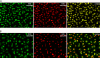
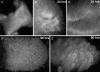
We have developed a method to detect DNA synthesis in proliferating cells, based on the incorporation of 5-ethynyl-2'-deoxyuridine (EdU) and its subsequent detection by a fluorescent azide through a Cu(I)-catalyzed [3 + 2] cycloaddition reaction ("click" chemistry). Detection of the EdU label is highly sensitive and can be accomplished in minutes. The small size of the fluorescent azides used for detection results in a high degree of specimen penetration, allowing the staining of whole-mount preparations of large tissue and organ explants. In contrast to BrdU, the method does not require sample fixation or DNA denaturation and permits good structural preservation. We demonstrate the use of the method in cultured cells and in the intestine and brain of whole animals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
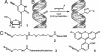

References
-
- Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218:474–475. - PubMed
-
- Waldman FM, et al. A comparison between bromodeoxyuridine and 3H thymidine labeling in human breast tumors. Mod Pathol. 1991;4:718–722. - PubMed
-
- Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci. 2002;3:65–71. - PubMed
-
- Tornoe CW, et al. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(i) -catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

