Dexamethasone-induced inositol 1,4,5-trisphosphate receptor elevation in murine lymphoma cells is not required for dexamethasone-mediated calcium elevation and apoptosis
- PMID: 18272518
- PMCID: PMC2447640
- DOI: 10.1074/jbc.M800269200
Dexamethasone-induced inositol 1,4,5-trisphosphate receptor elevation in murine lymphoma cells is not required for dexamethasone-mediated calcium elevation and apoptosis
Abstract
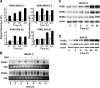
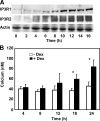
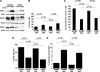
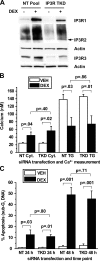
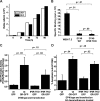
Glucocorticosteroid hormones, including dexamethasone, have diverse effects on immature lymphocyte function that ultimately lead to cell death. Previous studies established that glucocorticoid-induced alterations in intracellular calcium homeostasis promote apoptosis, but the mechanism by which glucocorticoids disrupt calcium homeostasis is unknown. Through gene expression array analysis, we found that dexamethasone induces a striking elevation of inositol 1,4,5-trisphosphate receptor (IP(3)R) levels in two murine lymphoma cell lines, WEHI7.2 and S49.A2. IP(3)R elevation was confirmed at both mRNA and protein levels. However, there was not a strong correlation between IP(3)R elevation and altered calcium homeostasis in terms of either kinetics or dose response. Moreover, IP(3)R knockdown, by either antisense or small interfering RNA, did not prevent either calcium disruption or apoptosis. Finally, DT40 lymphoma cells lacking all three IP(3)R isoforms were just as sensitive to dexamethasone-induced apoptosis as wild-type DT40 cells expressing all three IP(3)R isoforms. Thus, although alterations in intracellular calcium homeostasis contribute to glucocorticoid-induced apoptosis, these calcium alterations are not directly attributable to IP(3)R elevation.
Figures
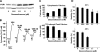

References
-
- Rhen, T., and Cidlowski, J. A. (2005) N. Engl. J. Med. 353 1711–1723 - PubMed
-
- Ashwell, J. D., Lu, F. W., and Vacchio, M. S. (2000) Annu. Rev. Immunol. 18 309–345 - PubMed
-
- Jondal, M., Pazirandeh, A., and Okret, S. (2004) Trends Immunol. 25 595–600 - PubMed
-
- Distelhorst, C. W. (2002) Cell Death Differ. 9 6–19 - PubMed
-
- Wyllie, A. H. (1980) Nature 284 555–556 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

