NMR and MD studies of the temperature-dependent dynamics of RNA YNMG-tetraloops
- PMID: 18272534
- PMCID: PMC2346598
- DOI: 10.1093/nar/gkm1183
NMR and MD studies of the temperature-dependent dynamics of RNA YNMG-tetraloops
Abstract
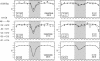
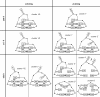
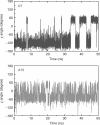
In a combined NMR/MD study, the temperature-dependent changes in the conformation of two members of the RNA YNMG-tetraloop motif (cUUCGg and uCACGg) have been investigated at temperatures of 298, 317 and 325 K. The two members have considerable different thermal stability and biological functions. In order to address these differences, the combined NMR/MD study was performed. The large temperature range represents a challenge for both, NMR relaxation analysis (consistent choice of effective bond length and CSA parameter) and all-atom MD simulation with explicit solvent (necessity to rescale the temperature). A convincing agreement of experiment and theory is found. Employing a principle component analysis of the MD trajectories, the conformational distribution of both hairpins at various temperatures is investigated. The ground state conformation and dynamics of the two tetraloops are indeed found to be very similar. Furthermore, both systems are initially destabilized by a loss of the stacking interactions between the first and the third nucleobase in the loop region. While the global fold is still preserved, this initiation of unfolding is already observed at 317 K for the uCACGg hairpin but at a significantly higher temperature for the cUUCGg hairpin.
Figures


References
-
- Al-Hashimi HM. Beyond static structures of RNA by NMR: folding, refolding, and dynamics at atomic resolution. Biopolymers. 2007;86:345–347. - PubMed
-
- Wenter P, Fürtig B, Hainard A, Schwalbe H, Pitsch S. A caged uridine for the selective preparation of an RNA fold and determination of its refolding kinetics by real-time NMR. Chem. Biochem. 2006;7:417–420. - PubMed
-
- Fürtig B, Buck J, Manoharan V, Bermel W, Jäschke A, Wenter P, Pitsch S, Schwalbe H. Time-resolved NMR studies of RNA folding. Biopolymers. 2007;27:360–383. - PubMed
-
- Al-Hashimi HM. Dynamics-based amplification of RNA function and its characterization by using NMR spectroscopy. Chem. Biochem. 2005;6:1506–1519. - PubMed
-
- Shajani Z, Varani G. NMR studies of dynamics in RNA and DNA by 13C relaxation. Biopolymers. 2007;86:348–359. - PubMed

