Brain tumor imaging in clinical trials
- PMID: 18272557
- PMCID: PMC8118884
- DOI: 10.3174/ajnr.A0963
Brain tumor imaging in clinical trials
Abstract
There are substantial challenges in the radiologic evaluation of tumor size during clinical trials, and it is important for neuroradiologists to have a firm understanding of these issues. This review will examine measurement approaches, response criteria, selection of lesions for measurement, technical imaging considerations, interval between tumor measurements and response confirmation, and validity of imaging as a measure of efficacy.
Figures
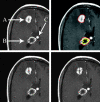
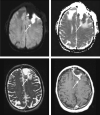
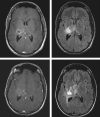
References
-
- Batchelor T, Stanley K, Andersen J. Clinical trials in neuro-oncology. Curr Opin Neurol 2001;14:689–94 - PubMed
-
- Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981;47:207–14 - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16 - PubMed
-
- Macdonald DR, Cascino TL, Schold SC, et al. Response criteria for phase II studies of malignant glioma. J Clin Oncol 1990;8:1277–80 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
