Standardized and highly efficient expansion of Epstein-Barr virus-specific CD4+ T cells by using virus-like particles
- PMID: 18272580
- PMCID: PMC2293016
- DOI: 10.1128/JVI.02227-07
Standardized and highly efficient expansion of Epstein-Barr virus-specific CD4+ T cells by using virus-like particles
Abstract
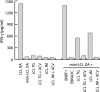
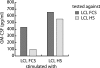
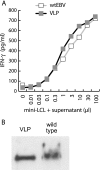
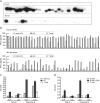
Epstein-Barr virus (EBV)-specific T-cell lines generated by repeated stimulation with EBV-immortalized lymphoblastoid B-cell lines (LCL) have been successfully used to treat EBV-associated posttransplant lymphoproliferative disease (PTLD) in hematopoietic stem cell transplant recipients. However, PTLD in solid-organ transplant recipients and other EBV-associated malignancies respond less efficiently to this adoptive T-cell therapy. LCL-stimulated T-cell preparations are polyclonal and contain CD4(+) and CD8(+) T cells, but the composition varies greatly between lines. Because T-cell lines with higher CD4(+) T-cell proportions show improved clinical efficacy, we assessed which factors might compromise the expansion of this T-cell population. Here we show that spontaneous virus production by LCL and, hence, the presentation of viral antigens varies intra- and interindividually and is further impaired by acyclovir treatment of LCL. Moreover, the stimulation of T cells with LCL grown in medium supplemented with fetal calf serum (FCS) caused the expansion of FCS-reactive CD4(+) T cells, whereas human serum from EBV-seropositive donors diminished viral antigen presentation. To overcome these limitations, we used peripheral blood mononuclear cells pulsed with nontransforming virus-like particles as antigen-presenting cells. This strategy facilitated the specific and rapid expansion of EBV-specific CD4(+) T cells and, thus, might contribute to the development of standardized protocols for the generation of T-cell lines with improved clinical efficacy.
Figures

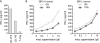
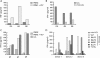
References
-
- Bevan, M. J. 2006. Cross-priming. Nat. Immunol. 7363-365. - PubMed
-
- Bollard, C. M., I. Kuehnle, A. Leen, C. M. Rooney, and H. E. Heslop. 2004. Adoptive immunotherapy for posttransplantation viral infections. Biol. Blood Marrow Transplant. 10143-155. - PubMed
-
- Chackerian, B. 2007. Virus-like particles: flexible platforms for vaccine development. Expert Rev. Vaccines 6381-390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

