Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens
- PMID: 18272687
- PMCID: PMC6671549
- DOI: 10.1523/JNEUROSCI.4275-07.2008
Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens
Abstract
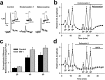
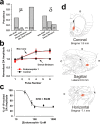
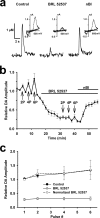
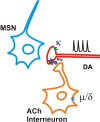
Behaviorally relevant stimuli prompt midbrain dopamine (DA) neurons to switch from tonic to burst firing patterns. Similar shifts to burst activity are thought to contribute to the addictive effects of opiates and nicotine. The nucleus accumbens DA overflow produced by these drugs is a key element in their pathological effects. Using electrochemical techniques in brain slices, we explored the effects of opioids on single-spike and burst stimuli-evoked DA overflow in the dorsal and ventral striatum. In specific subregions of the nucleus accumbens, mu-opioids inhibit DA overflow elicited with single-spike stimuli while leaving that produced by burst stimuli unaffected. This is similar to published effects of nicotinic receptor blockade or desensitization, and is mediated by opioid receptor-induced inhibition of cholinergic interneurons. Whereas delta-opioids have similar effects, kappa-opioids inhibit evoked DA overflow throughout the striatum in a manner that is not overcome with high-frequency stimuli. These observations reveal remarkable mechanistic overlap between the effects of nicotine and opiates within the dopamine reward pathway.
Figures



References
-
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31:1371–1381. - PubMed
-
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. - PubMed
-
- Brundege JM, Williams JT. Differential modulation of nucleus accumbens synapses. J Neurophysiol. 2002;88:142–151. - PubMed
-
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
