Rapid antimicrobial susceptibility determination of uropathogens in clinical urine specimens by use of ATP bioluminescence
- PMID: 18272708
- PMCID: PMC2292911
- DOI: 10.1128/JCM.02036-07
Rapid antimicrobial susceptibility determination of uropathogens in clinical urine specimens by use of ATP bioluminescence
Abstract
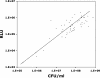
We describe the first direct testing of the antimicrobial susceptibilities of bacterial pathogens in human clinical fluid samples by the use of ATP bioluminescence. We developed an ATP bioluminescence assay that eliminates somatic sources of ATP to selectively quantify the bacterial load in clinical urine specimens with a sensitivity of <1,000 CFU per milliliter. There was a log-log relationship between light emission and the numbers of CFU in clinical urine specimens. A clinical study was performed to evaluate the accuracy of the ATP bioluminescence assay for determination of the antimicrobial susceptibilities of uropathogens in clinical urine specimens tested in a blinded manner. ATP bioluminescent bacterial density quantitation was used to determine the inoculation volume in growth medium with and without antibiotics. After incubation at 37 degrees C for 120 min, the ATP bioluminescence assay was repeated to evaluate the uropathogen response to antibiotics. The ability of the ATP bioluminescence assay to discriminate between antimicrobial susceptibility and resistance was determined by comparison of the results obtained by the ATP bioluminescence assay with the results obtained by standard clinical microbiology methods. Receiver operator characteristic curves were used to determine the optimal threshold for discriminating between susceptibility and resistance. Susceptibility and resistance were correctly predicted in 87% and 95% of cases, respectively, for an overall unweighted accuracy of 91%, when the results were stratified by antibiotic. For samples in which the pathogen was susceptible, the accuracy improved to 95% when the results for samples with less than a 25-fold increase in the amount of bacterial ATP in the medium without antibiotics were excluded. These data indicate that a rapid bioluminescent antimicrobial susceptibility assay may be useful for the management of urinary tract infections.
Figures

References
-
- Chappelle, E. W., and G. V. Levin. 1968. Use of the firefly bioluminescent reaction for rapid detection and counting of bacteria. Biochem. Med. 241-52.
-
- Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, seventh edition. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Conn, R. B., P. Charache, and E. W. Chappelle. 1975. Limits of applicability of the firefly luminescence ATP assay for the detection of bacteria in clinical specimens. Am. J. Clin. Pathol. 63493-501. - PubMed
-
- Czaja, C. A., D. Scholes, T. M. Hooton, and W. E. Stamm. 2007. Population-based epidemiologic analysis of acute pyelonephritis. Clin. Infect. Dis. 45273-280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

