Na/H exchange regulatory factor 1, a novel AKT-associating protein, regulates extracellular signal-regulated kinase signaling through a B-Raf-mediated pathway
- PMID: 18272783
- PMCID: PMC2291404
- DOI: 10.1091/mbc.e07-11-1114
Na/H exchange regulatory factor 1, a novel AKT-associating protein, regulates extracellular signal-regulated kinase signaling through a B-Raf-mediated pathway
Abstract
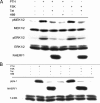
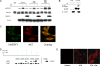
Na/H exchange regulatory factor 1 (NHERF1) is a scaffolding protein that regulates signaling and trafficking of several G protein-coupled receptors (GPCRs), including the parathyroid hormone receptor (PTH1R). GPCRs activate extracellular signal-regulated kinase (ERK)1/2 through different mechanisms. Here, we characterized NHERF1 regulation of PTH1R-stimulated ERK1/2. Parathyroid hormone (PTH) stimulated ERK1/2 phosphorylation by a protein kinase A (PKA)-dependent, but protein kinase C-, cyclic adenosine 5'-monophosphate-, and Rap1-independent pathway in Chinese hamster ovary cells stably transfected with the PTH1R and engineered to express NHERF1 under the control of tetracycline. NHERF1 blocked PTH-induced ERK1/2 phosphorylation downstream of PKA. This suggested that NHERF1 inhibitory effects on ERK1/2 occur at a postreceptor locus. Forskolin activated ERK1/2, and this effect was blocked by NHERF1. NHERF1 interacted with AKT and inhibited ERK1/2 activation by decreasing the stimulatory effect of 14-3-3 binding to B-Raf, while increasing the inhibitory influence of AKT negative regulation on ERK1/2 activation. This novel regulatory mechanism provides a new model by which cytoplasmic adapter proteins modulate ERK1/2 activation through a receptor-independent mechanism involving B-Raf.
Figures



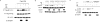
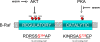
References
-
- Ahmed I., Gesty-Palmer D., Drezner M. K., Luttrell L. M. Transactivation of the epidermal growth factor receptor mediates parathyroid hormone and prostaglandin F2α-stimulated mitogen-activated protein kinase activation in cultured transgenic murine osteoblasts. Mol. Endocrinol. 2003;17:1607–1621. - PubMed
-
- Aitken A., Baxter H., Dubois T., Clokie S., Mackie S., Mitchell K., Peden A., Zemlickova E. Specificity of 14-3-3 isoform dimer interactions and phosphorylation. Biochem. Soc. Trans. 2002;30:351–360. - PubMed
-
- Bretscher A., Chambers D., Nguyen R., Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu. Rev. Cell Dev. Biol. 2000;16:113–143. - PubMed
-
- Bretscher A., Edwards K., Fehon R. G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002;3:586–599. - PubMed
-
- Calipel A., Mouriaux F., Glotin A. L., Malecaze F., Faussat A. M., Mascarelli F. Extracellular signal-regulated kinase-dependent proliferation is mediated through the protein kinase A/B-Raf pathway in human uveal melanoma cells. J. Biol. Chem. 2006;281:9238–9250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

