Dynamic imaging of fibrin network formation correlated with other measures of polymerization
- PMID: 18272815
- PMCID: PMC2384121
- DOI: 10.1182/blood-2007-08-105247
Dynamic imaging of fibrin network formation correlated with other measures of polymerization
Abstract
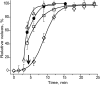
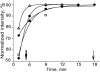
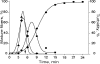
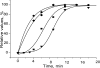
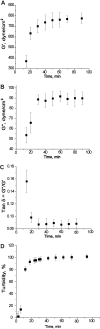
Using deconvolution microscopy, we visualized in real time fibrin network formation in the hydrated state. Individual mobile fibers were observed before the gel point determined by eye. After gelation, an initial fibrin network was seen, which evolved over time by addition of new fibers and elongation and branching of others. Furthermore, some fibers in the network moved for a time. We quantified network formation by number of branch points, and longitudinal and lateral growth of fibers. Eighty percent of branch points were formed, and 70% of all fibers reached their maximum length at the gel point. In contrast, at the gel point, fiber diameter, measured as fluorescence intensity, was less than 25% and turbidity was less than 15% of the maximum values of the fully formed clot. The cumulative percentage of fibers reaching their final length and the number of branch points attained maximum values at 60% of maximum turbidity. Lateral fiber growth reached a plateau at the same time as turbidity. Measurements of clot mechanical properties revealed that the clots achieved maximum stiffness and minimum plasticity after the structural parameters reached their maxima. These results provide new information on the relative time sequence of events during fibrin network formation.
Figures


Comment in
-
Fibrin formation on fast forward.Blood. 2008 May 15;111(10):4839. doi: 10.1182/blood-2008-02-140913. Blood. 2008. PMID: 18467607
References
-
- Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–299. - PubMed
-
- Doolittle RF. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. - PubMed
-
- Cierniewski CS, Kloczewiak M, Budzynski AZ. Expression of primary polymerization sites in the D domain of human fibrinogen depends on intact conformation. J Biol Chem. 1986;261:9116–9121. - PubMed
-
- Weisel JW, Phillips GN, Jr, Cohen C. The structure of fibrinogen and fibrin: II, architecture of the fibrin clot. Ann N Y Acad Sci. 1983;408:367–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

