Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology
- PMID: 18272828
- PMCID: PMC2386709
- DOI: 10.2215/CJN.03630807
Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology
Abstract
Background and objectives: Peritoneal interstitial fibrosis and hyalinizing vasculopathy were induced by peritoneal dialysis and other associated conditions (e.g., uremia). A quantitative method for peritoneal biopsy evaluation is required to investigate possible causative factors and severity of the peritoneal dialysis-related peritoneal alterations.
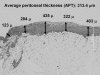
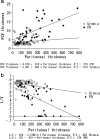
Design, setting, participants, & measurements: Peritoneal biopsy specimens from 173 uremic (before peritoneal dialysis) and 80 peritoneal dialysis patients with or without impaired ultrafiltration capacity were evaluated by average peritoneal thickness of submesothelial compact zone measured at five randomly selected points of peritoneum and by lumen/vessel diameter ratio at postcapillary venule.
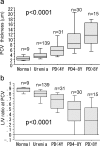
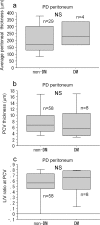
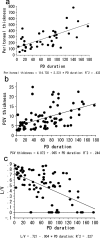
Results: The average peritoneal thickness was increased in uremic patients and progressively thickened as the duration of peritoneal dialysis prolonged. The lumen/vessel diameter ratio was lower in uremia than normal and progressively decreased as the duration of peritoneal dialysis prolonged. In pre-peritoneal dialysis peritoneum, patients with diabetes showed significant decrease in lumen/vessel diameter ratio compared with patients without diabetes. The average peritoneal thickness was significantly higher in patients with impaired ultrafiltration capacity than in patients with maintained ultrafiltration capacity; however, no significant difference was observed in the postcapillary venule thickness and lumen/vessel diameter ratio between the two groups.
Conclusions: The average peritoneal thickness and lumen/vessel diameter ratio were useful morphologic parameters to quantify the severity of the peritoneal alterations in uremic and peritoneal dialysis patients. Uremia and diabetes had an impact on the pathogenesis of peritoneal sclerosis in pre-peritoneal dialysis peritoneum. Peritoneal dialysis treatment itself had a much stronger impact on the progression of peritoneal sclerosis.
Figures






References
-
- Di Paolo N, Sacchi G, De Mia M, Gaggiotti E, Capotondo L, Rossi P, Bernini M, Pucci AM, Ibba L, Sabatelli P, Alessandrini C: Morphology of the peritoneal membrane during continuous ambulatory peritoneal dialysis. Nephron 44: 204–211, 1986 - PubMed
-
- Gotloib L, Shostak A: Ultrastructural morphology of the peritoneum: New findings and speculations on transfer of solutes and water during peritoneal dialysis. Perit Dial Bull 7: 119–129, 1987
-
- Dobbie JW: Morphology of the peritoneum in CAPD. Blood Purif 7: 74–85, 1989 - PubMed
-
- Honda K, Nitta K, Horita S, Yumura W, Nihei H: Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron 72: 171–176, 1996 - PubMed
-
- Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, Krediet RT: Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 19: 517–525, 1999 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

