Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure
- PMID: 18274669
- PMCID: PMC2242621
- DOI: 10.1172/JCI33871
Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure
Abstract
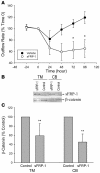
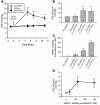
Elevated intraocular pressure (IOP) is the principal risk factor for glaucoma and results from excessive impedance of the fluid outflow from the eye. This abnormality likely originates from outflow pathway tissues such as the trabecular meshwork (TM), but the associated molecular etiology is poorly understood. We discovered what we believe to be a novel role for secreted frizzled-related protein-1 (sFRP-1), an antagonist of Wnt signaling, in regulating IOP. sFRP1 was overexpressed in human glaucomatous TM cells. Genes involved in the Wnt signaling pathway were expressed in cultured TM cells and human TM tissues. Addition of recombinant sFRP-1 to ex vivo perfusion-cultured human eyes decreased outflow facility, concomitant with reduced levels of beta-catenin, the Wnt signaling mediator, in the TM. Intravitreal injection of an adenoviral vector encoding sFRP1 in mice produced a titer-dependent increase in IOP. Five days after vector injection, IOP increased 2 fold, which was significantly reduced by topical ocular administration of an inhibitor of a downstream suppressor of Wnt signaling. Thus, these data indicate that increased expression of sFRP1 in the TM appears to be responsible for elevated IOP in glaucoma and restoring Wnt signaling in the TM may be a novel disease intervention strategy for treating glaucoma.
Figures



References
-
- Quigley H.A. The search for glaucoma genes — implications for pathogenesis and disease detection. N. Engl. J. Med. 1998;338:1063–1064. - PubMed
-
- Quigley H.A. Open-angle glaucoma. N. Engl. J. Med. 1993;328:1097–1106. - PubMed
-
- Kass M.A., et al. 2002The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 120701–713; discussion 829–830. . - PubMed
-
- The AGIS Investigators. . The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am. J. Ophthalmol. 2000;130:429–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

