The peroxisome: still a mysterious organelle
- PMID: 18274771
- PMCID: PMC2668598
- DOI: 10.1007/s00418-008-0396-9
The peroxisome: still a mysterious organelle
Abstract
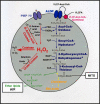
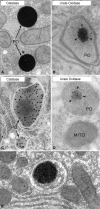
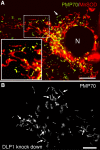
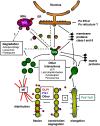
More than half a century of research on peroxisomes has revealed unique features of this ubiquitous subcellular organelle, which have often been in disagreement with existing dogmas in cell biology. About 50 peroxisomal enzymes have so far been identified, which contribute to several crucial metabolic processes such as beta-oxidation of fatty acids, biosynthesis of ether phospholipids and metabolism of reactive oxygen species, and render peroxisomes indispensable for human health and development. It became obvious that peroxisomes are highly dynamic organelles that rapidly assemble, multiply and degrade in response to metabolic needs. However, many aspects of peroxisome biology are still mysterious. This review addresses recent exciting discoveries on the biogenesis, formation and degradation of peroxisomes, on peroxisomal dynamics and division, as well as on the interaction and cross talk of peroxisomes with other subcellular compartments. Furthermore, recent advances on the role of peroxisomes in medicine and in the identification of novel peroxisomal proteins are discussed.
Figures

References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC554876', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC554876/'}, {'type': 'PubMed', 'value': '15795412', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15795412/'}]}
- Adams LA, Angulo P, Lindor KD (2005) Nonalcoholic fatty liver disease. CMAJ 172:899–905 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/cne.21448', 'is_inner': False, 'url': 'https://doi.org/10.1002/cne.21448'}, {'type': 'PubMed', 'value': '17729295', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17729295/'}]}
- Ahlemeyer B, Neubert I, Kovacs WJ, Baumgart-Vogt E (2007) Differential expression of peroxisomal matrix and membrane proteins during postnatal development of mouse brain. J Comp Neurol 505:1–17 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '3080517', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/3080517/'}]}
- Angermuller S, Fahimi HD (1986) Ultrastructural cytochemical localization of uricase in peroxisomes of rat liver. J Histochem Cytochem 34:159–165 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/j.bbamcr.2006.08.044', 'is_inner': False, 'url': 'https://doi.org/10.1016/j.bbamcr.2006.08.044'}, {'type': 'PubMed', 'value': '17045662', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17045662/'}]}
- Antonenkov VD, Hiltunen JK (2006) Peroxisomal membrane permeability and solute transfer. Biochim Biophys Acta 1763:1697–1706 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1083/jcb.137.1.37', 'is_inner': False, 'url': 'https://doi.org/10.1083/jcb.137.1.37'}, {'type': 'PMC', 'value': 'PMC2139849', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2139849/'}, {'type': 'PubMed', 'value': '9105035', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9105035/'}]}
- Bassnett S, Mataic D (1997) Chromatin degradation in differentiating fiber cells of the eye lens. J Cell Biol 137:37–49 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

