Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast huisgen cycloadditions
- PMID: 18275058
- PMCID: PMC2835304
- DOI: 10.1002/anie.200705456
Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast huisgen cycloadditions
Figures
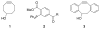
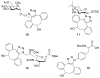
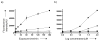

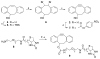
Comment in
-
Copper-free azide-alkyne cycloadditions: new insights and perspectives.Angew Chem Int Ed Engl. 2008;47(12):2182-4. doi: 10.1002/anie.200705365. Angew Chem Int Ed Engl. 2008. PMID: 18264961 No abstract available.
References
-
- Kolb HC, Sharpless KB. Drug Dis Today. 2003;8:1128–1137. - PubMed
-
- Dedola S, Nepogodiev SA, Field RA. Org Biomol Chem. 2007;5:1006–1017. - PubMed
-
- Moses JE, Moorhouse AD. Chem Soc Rev. 2007;36:1249–1262. - PubMed
-
- Nandivada H, Jiang XW, Lahann J. Adv Mater. 2007;19:2197–2208.
-
- Wu P, Fokin VV. Aldrichim Acta. 2007;40:7–17.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

