Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle
- PMID: 18276596
- PMCID: PMC2442306
- DOI: 10.1074/jbc.M708839200
Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle
Abstract
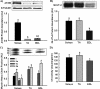
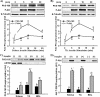
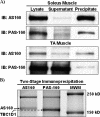
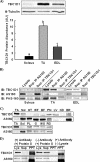
The Akt substrate of 160 kDa (AS160) is phosphorylated on Akt substrate (PAS) motifs in response to insulin and contraction in skeletal muscle, regulating glucose uptake. Here we discovered a dissociation between AS160 protein expression and apparent AS160 PAS phosphorylation among soleus, tibialis anterior, and extensor digitorum longus muscles. Immunodepletion of AS160 in tibialis anterior muscle lysates resulted in minimal depletion of the PAS band at 160 kDa, suggesting the presence of an additional PAS immunoreactive protein. By immunoprecipitation and mass spectrometry, we identified this protein as the AS160 paralog TBC1D1, an obesity candidate gene regulating GLUT4 translocation in adipocytes. TBC1D1 expression was severalfold higher in skeletal muscles compared with all other tissues and was the dominant protein detected by the anti-PAS antibody at 160 kDa in tibialis anterior and extensor digitorum longus but not soleus muscles. In vivo stimulation by insulin, contraction, and the AMP-activated protein kinase (AMPK) activator AICAR increased TBC1D1 PAS phosphorylation. Using mass spectrometry on TBC1D1 from mouse skeletal muscle, we identified several novel phosphorylation sites on TBC1D1 and found the majority were consensus or near consensus sites for AMPK. Semiquantitative analysis of spectra suggested that AICAR caused greater overall phosphorylation of TBC1D1 sites compared with insulin. Purified Akt and AMPK phosphorylated TBC1D1 in vitro, and AMPK, but not Akt, reduced TBC1D1 electrophoretic mobility. TBC1D1 is a major PAS immunoreactive protein in skeletal muscle that is phosphorylated in vivo by insulin, AICAR, and contraction. Both Akt and AMPK phosphorylate TBC1D1, but AMPK may be the more robust regulator.
Figures



References
-
- Richter, E. A., Nielsen, J. N., Jorgensen, S. B., Frosig, C., Birk, J. B., and Wojtaszewski, J. F. (2004) Proc. Nutr. Soc. 63 211-216 - PubMed
-
- Jessen, N., and Goodyear, L. J. (2005) J. Appl. Physiol. 99 330-337 - PubMed
-
- Kennedy, J. W., Hirshman, M. F., Gervino, E. V., Ocel, J. V., Forse, R. A., Hoenig, S. J., Aronson, D., Goodyear, L. J., and Horton, E. S. (1999) Diabetes 48 1192-1197 - PubMed
-
- Kramer, H. F., Witczak, C. A., Taylor, E. B., Fujii, N., Hirshman, M. F., and Goodyear, L. J. (2006) J. Biol. Chem. 281 31478-31485 - PubMed
-
- Sano, H., Kane, S., Sano, E., Miinea, C. P., Asara, J. M., Lane, W. S., Garner, C. W., and Lienhard, G. E. (2003) J. Biol. Chem. 278 14599-14602 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

