Efficient Ca2+ buffering in fast-spiking basket cells of rat hippocampus
- PMID: 18276734
- PMCID: PMC2465201
- DOI: 10.1113/jphysiol.2007.147298
Efficient Ca2+ buffering in fast-spiking basket cells of rat hippocampus
Abstract
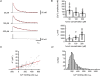
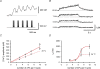
Fast-spiking parvalbumin-expressing basket cells (BCs) represent a major type of inhibitory interneuron in the hippocampus. These cells inhibit principal cells in a temporally precise manner and are involved in the generation of network oscillations. Although BCs show a unique expression profile of Ca(2+)-permeable receptors, Ca(2+)-binding proteins and Ca(2+)-dependent signalling molecules, physiological Ca(2+) signalling in these interneurons has not been investigated. To study action potential (AP)-induced dendritic Ca(2+) influx and buffering, we combined whole-cell patch-clamp recordings with ratiometric Ca(2+) imaging from the proximal apical dendrites of rigorously identified BCs in acute slices, using the high-affinity Ca(2+) indicator fura-2 or the low-affinity dye fura-FF. Single APs evoked dendritic Ca(2+) transients with small amplitude. Bursts of APs evoked Ca(2+) transients with amplitudes that increased linearly with AP number. Analysis of Ca(2+) transients under steady-state conditions with different fura-2 concentrations and during loading with 200 microm fura-2 indicated that the endogenous Ca(2+)-binding ratio was approximately 200 (kappa(S) = 202 +/- 26 for the loading experiments). The peak amplitude of the Ca(2+) transients measured directly with 100 microm fura-FF was 39 nm AP(-1). At approximately 23 degrees C, the decay time constant of the Ca(2+) transients was 390 ms, corresponding to an extrusion rate of approximately 600 s(-1). At 34 degrees C, the decay time constant was 203 ms and the corresponding extrusion rate was approximately 1100 s(-1). At both temperatures, continuous theta-burst activity with three to five APs per theta cycle, as occurs in vivo during exploration, led to a moderate increase in the global Ca(2+) concentration that was proportional to AP number, whereas more intense stimulation was required to reach micromolar Ca(2+) concentrations and to shift Ca(2+) signalling into a non-linear regime. In conclusion, dentate gyrus BCs show a high endogenous Ca(2+)-binding ratio, a small AP-induced dendritic Ca(2+) influx, and a relatively slow Ca(2+) extrusion. These specific buffering properties of BCs will sharpen the time course of local Ca(2+) signals, while prolonging the decay of global Ca(2+) signals.
Figures





Comment in
-
Details of Ca2+ dynamics matter.J Physiol. 2008 Apr 15;586(8):2031. doi: 10.1113/jphysiol.2008.153080. J Physiol. 2008. PMID: 18413335 Free PMC article. No abstract available.
References
-
- Aponte Y, Lien CC, Bischofberger J, Jonas P. Dendritic calcium signaling in fast-spiking hippocampal basket cells. Abstr Soc Neurosci. 2006a;337.12
-
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

