Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis
- PMID: 18276777
- PMCID: PMC2258272
- DOI: 10.2353/ajpath.2008.070918
Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis
Abstract
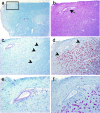
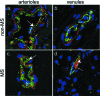
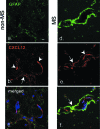
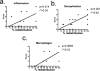
Dysregulation of blood-brain barrier (BBB) function and transendothelial migration of leukocytes are essential components of the development and propagation of active lesions in multiple sclerosis (MS). Animal studies indicate that polarized expression of the chemokine CXCL12 at the BBB prevents leukocyte extravasation into the central nervous system (CNS) and that disruption of CXCL12 polarity promotes entry of autoreactive leukocytes and inflammation. In the present study, we examined expression of CXCL12 and its receptor, CXCR4, within CNS tissues from MS and non-MS patients. Immunohistochemical analysis of CXCL12 expression at the BBB revealed basolateral localization in tissues derived from non-MS patients and at uninvolved sites in tissues from MS patients. In contrast, within active MS lesions, CXCL12 expression was redistributed toward vessel lumena and was associated with CXCR4 activation in infiltrating leukocytes, as revealed by phospho-CXCR4-specific antibodies. Quantitative assessment of CXCL12 expression by the CNS microvasculature established a positive correlation between CXCL12 redistribution, leukocyte infiltration, and severity of histological disease. These results suggest that CXCL12 normally functions to localize infiltrating leukocytes to perivascular spaces, preventing CNS parenchymal infiltration. In the patient cohort studied, altered patterns of CXCL12 expression at the BBB were specifically associated with MS, possibly facilitating trafficking of CXCR4-expressing mononuclear cells into and out of the perivascular space and leading to progression of disease.
Figures



References
-
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. - PubMed
-
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis: the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. - PubMed
-
- Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. - PubMed
-
- Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. - PubMed
-
- Durán I, Martinez-Caceres EM, Rio J, Barbera N, Marzo ME, Montalban X. Immunological profile of patients with primary progressive multiple sclerosis: expression of adhesion molecules. Brain. 1999;122:2297–2307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

