Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice
- PMID: 18276942
- PMCID: PMC2383993
- DOI: 10.1164/rccm.200708-1243OC
Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice
Abstract
Rationale: Infection with rhinovirus (RV) triggers exacerbations of asthma and chronic obstructive lung disease.
Objectives: We sought to develop a mouse model of RV employing RV1B, a minor group serotype that binds to the low-density lipoprotein receptor.
Methods: C57BL/6 mice were inoculated intranasally with RV1B, replication-deficient ultraviolet (UV)-irradiated RV1B, or RV39, a major group virus.
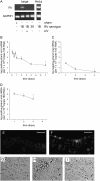
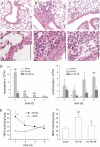
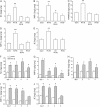
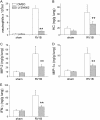
Measurements and main results: Viral RNA was present in the lungs of RV1B-treated mice, but not in those exposed to UV-irradiated RV1B or RV39. Lung homogenates of RV-treated mice contained infectious RV 4 days after inoculation. RV1B exposure induced neutrophilic and lymphocytic airway inflammation, as well as increased lung expression of KC, macrophage-inflammatory protein-2, and IFN-alpha and IFN-beta. RV1B-exposed mice showed airway hyperresponsiveness 1 and 4 days after inoculation. UV-irradiated RV1B induced modest neutrophilic airway inflammation and hyperresponsiveness 1 day after exposure. Both RV1B and UV-irradiated RV1B, but not RV39, increased lung phosphorylation of Akt. Confocal immunofluorescence showed colocalization of RV1B and phospho-Akt in the airway epithelium. Finally, pretreatment with the phosphatidylinositol 3-kinase inhibitor LY294002 attenuated chemokine production and neutrophil infiltration.
Conclusions: We conclude that RV1B induces airway inflammation in vivo. Evidence is presented that viral replication occurs in vivo and is required for maximal responses. On the other hand, viral replication was not required for a subset of RV-induced responses, including neutrophilic inflammation, airway hyperresponsiveness, and Akt phosphorylation. Finally, phosphatidylinositol 3-kinase/Akt signaling is required for maximal RV1B-induced airway neutrophilic inflammation, likely via its essential role in virus internalization.
Figures


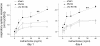

References
-
- Kling S, Donninger H, Williams Z, Vermeulen J, Weinberg E, Latiff K, Ghildyal R, Bardin P. Persistence of rhinovirus RNA after asthma exacerbation in children. Clin Exp Allergy 2005;35:672–678. - PubMed
-
- Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:167–173. - PubMed
-
- Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1618–1623. - PubMed
-
- Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, Meyer J, Lackie PM, Sanderson G, Holgate ST, et al. Rhinoviruses infect the lower airways. J Infect Dis 2000;181:1875–1884. - PubMed

