Risk factors for late postnatal transmission of human immunodeficiency virus type 1 in sub-Saharan Africa
- PMID: 18277935
- PMCID: PMC2730543
- DOI: 10.1097/INF.0b013e31815b4960
Risk factors for late postnatal transmission of human immunodeficiency virus type 1 in sub-Saharan Africa
Abstract
Background: We conducted secondary data analyses of a clinical trial (HIVNET 024) to assess risk factors for late postnatal transmission (LPT) of human immunodeficiency virus type 1 (HIV-1) through breast-feeding.
Methods: Data regarding live born, singleton infants of HIV-1-infected mothers were analyzed. The timing of HIV-1 transmission through 12 months after birth was defined as: in utero (positive HIV-1 RNA results at birth), perinatal/early postnatal (negative results at birth, positive at 4-6 week visit), or LPT (negative results through the 4-6 week visit, but positive assays thereafter through the 12-month visit). HIV-1-uninfected infants were those with negative HIV-1 enzyme immunoassay results at 12 months of age, or infants with negative HIV-1 RNA results throughout follow-up.
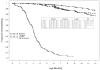
Results: Of 2292 HIV-1-infected enrolled women, 2052 mother/infant pairs met inclusion criteria. Of 1979 infants with HIV-1 tests, 404 were HIV-1-infected, and 382 had known timing of infection (LPT represented 22% of transmissions). Further analyses of LPT included infants who were breast-feeding at the 4-6 week visit (with negative HIV-1 results at that visit) revealed 6.9% of 1317 infants acquired HIV-1 infection through LPT by 12 months of age. More advanced maternal HIV-1 disease at enrollment (lower CD4 counts, higher plasma viral loads) were the factors associated with LPT in adjusted analyses.
Conclusions: In this breast-feeding population, 6.9% of infants uninfected at 6 weeks of age acquired HIV-1 infection by 12 months. Making interventions to decrease the risk of LPT of HIV-1 available and continuing research regarding the mechanisms of LPT (so as to develop improved interventions to reduce such transmission) remain essential.
Figures
References
-
- Read JS. Prevention of mother-to-child transmission of HIV. In: Zeichner SL, Read JS, editors. Textbook of Pediatric HIV Care. Cambridge, England: Cambridge University Press; 2005. pp. 111–33.
-
- The Working Group on Mother-to-Child Transmission of HIV. Rates of mother-to-child transmission of HIV-1 in Africa, America, and Europe: results from 13 perinatal studies. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8(5):506–510. - PubMed
-
- The Breastfeeding and HIV International Transmission Study (BHITS) Group. Late postnatal transmission of HIV-1 in breastfed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. - PubMed
-
- Taha T, Hoffman I, Fawzi W, Brown E, Read JS, Valentine M, et al. A phase III clinical trial of antibiotics to reduce chorioamnionitis-related perinatal HIV-1 transmission (HPTN024) AIDS. 2006;20:1313–1321. - PubMed
-
- Guay L, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354(9181):795–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI068619/AI/NIAID NIH HHS/United States
- N01-AI-35173-117/412/AI/NIAID NIH HHS/United States
- U01-AI-48005/AI/NIAID NIH HHS/United States
- U01 AI048006/AI/NIAID NIH HHS/United States
- U01 AI048005/AI/NIAID NIH HHS/United States
- U01-AI-48006/AI/NIAID NIH HHS/United States
- U01 AI047972/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- P30 AI-50410/AI/NIAID NIH HHS/United States
- N01 AI045200/AI/NIAID NIH HHS/United States
- N01 AI035173/AI/NIAID NIH HHS/United States
- U01-AI-47972/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous