Versatility of biological non-heme Fe(II) centers in oxygen activation reactions
- PMID: 18277980
- PMCID: PMC2720164
- DOI: 10.1038/nchembio.71
Versatility of biological non-heme Fe(II) centers in oxygen activation reactions
Abstract
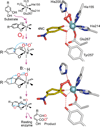
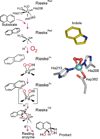
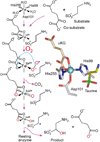
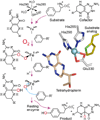
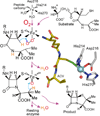
Oxidase and oxygenase enzymes allow the use of relatively unreactive O2 in biochemical reactions. Many of the mechanistic strategies used in nature for this key reaction are represented within the 2-histidine-1-carboxylate facial triad family of non-heme Fe(II)-containing enzymes. The open face of the metal coordination sphere opposite the three endogenous ligands participates directly in the reaction chemistry. Here, data from several studies are presented showing that reductive O2 activation within this family is initiated by substrate (and in some cases cosubstrate or cofactor) binding, which then allows coordination of O2 to the metal. From this starting point, the O2 activation process and the reactions with substrates diverge broadly. The reactive species formed in these reactions have been proposed to encompass four oxidation states of iron and all forms of reduced O2 as well as several of the reactive oxygen species that derive from O-O bond cleavage.
Figures

References
-
- Ozer A, Bruick RK. Non-heme dioxygenases: cellular sensors and regulators jelly rolled into one? Nat. Chem. Biol. 2007;3:144–153. - PubMed
-
- Kirk TK. In: Degradation of lignin. in Microbial Degradation of Organic Compounds. Gibson DT, editor. Vol. 13. New York, New York: Marcel Dekker, Inc; 1984. pp. 399–438.
-
- Hakemian AS, Rosenzweig AC. The Biochemistry of Methane Oxidation. Annu. Rev. Biochem. 2007;76:223–241. - PubMed
-
- Gibson DT, Parales RE. Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr. Opin. Biotechnol. 2000;11:236–243. - PubMed
-
- Baldwin JE, Abraham E. Biosynthesis of penicillins and cephalosporins. Nat. Prod. Rep. 1988;5:129–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

