Nickel compounds induce histone ubiquitination by inhibiting histone deubiquitinating enzyme activity
- PMID: 18279901
- PMCID: PMC2424130
- DOI: 10.1016/j.taap.2007.12.015
Nickel compounds induce histone ubiquitination by inhibiting histone deubiquitinating enzyme activity
Abstract
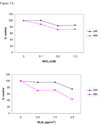
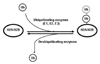
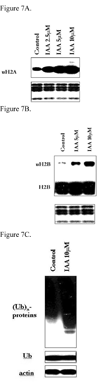
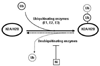
Nickel (Ni) compounds are known carcinogens but underlying mechanisms are not clear. Epigenetic changes are likely to play an important role in nickel ion carcinogenesis. Previous studies have shown epigenetic effects of nickel ions, including the loss of histone acetylation and a pronounced increase in dimethylated H3K9 in nickel-exposed cells. In this study, we demonstrated that both water-soluble and insoluble nickel compounds induce histone ubiquitination (uH2A and uH2B) in a variety of cell lines. Investigations of the mechanism by which nickel increases histone ubiquitination in cells reveal that nickel does not affect cellular levels of the substrates of this modification, i.e., ubiquitin, histones, and other non-histone ubiquitinated proteins. In vitro ubiquitination and deubiquitination assays have been developed to further investigate possible effects of nickel on enzymes responsible for histone ubiquitination. Results from the in vitro assays demonstrate that the presence of nickel did not affect the levels of ubiquitinated histones in the ubiquitinating assay. Instead, the addition of nickel significantly prevents loss of uH2A and uH2B in the deubiquitinating assay, suggesting that nickel-induced histone ubiquitination is the result of inhibition of (a) putative deubiquitinating enzyme(s). Additional supporting evidence comes from the comparison of the response to nickel ions with a known deubiquitinating enzyme inhibitor, iodoacetamide (IAA). This study is the first to demonstrate such effects of nickel ions on histone ubiquitination. It also sheds light on the possible mechanisms involved in altering the steady state of this modification. The study provides further evidence that supports the notion that nickel ions alter epigenetic homeostasis in cells, which may lead to altered programs of gene expression and carcinogenesis.
Figures








References
-
- Biedermann KA, Landolph JR. Induction of anchorage independence in human diploid foreskin fibroblasts by carcinogenic metal salts. Cancer Res. 1987;47:3815–3823. - PubMed
-
- Biggart NW, Costa M. Assessment of the uptake and mutagenicity of nickel chloride in salmonella tester strains. Mutat Res. 1986;175:209–215. - PubMed
-
- Broday L, Cai J, Costa M. Nickel enhances telomeric silencing in Saccharomyces cerevisiae. Mutat Res. 1999;440:121–130. - PubMed
-
- Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20:845–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

