Molecular and functional characterization of a tandem-repeat galectin from the freshwater snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni
- PMID: 18280060
- PMCID: PMC2423817
- DOI: 10.1016/j.gene.2008.01.003
Molecular and functional characterization of a tandem-repeat galectin from the freshwater snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni
Abstract
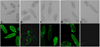
In the present study, a tandem-repeat type galectin was characterized from an embryonic cell line (Bge) and circulating hemocytes of the snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni. The predicted B. glabrata galectin (BgGal) protein of 32 kDa possessed 2 carbohydrate recognition domains, each displaying 6 of 8 conserved amino acids involved in galactoside-binding activity. A recombinant BgGal (rBgGal) demonstrated hemagglutinating activity against rabbit erythrocytes, which was specifically inhibited by galactose-containing sugars (lacNAc/lac>galNAc/gal). Although native galectin was immunolocalized in the cytoplasm of Bge cells and the plasma membrane of a subset of snail hemocytes (60%), it was not detected in cell-free plasma by Western blot analysis. The findings that rBgGal selectively recognizes the schistosome-related sugar, lacNAc, and strongly binds to hemocytes and the tegument of S. mansoni sporocysts in a sugar-inhibitable fashion suggest that hemocyte-bound galectin may be serving as a pattern recognition receptor for this, or other pathogens possessing appropriate sugar ligands. Based on molecular and functional features, BgGal represents an authentic galectin, the first to be fully characterized in the medically-important molluscan Class Gastropoda.
Figures







References
-
- Acosta-Rodriguez EV, Montes CL, Motran CC, Zuniga EI, Liu F-T, Rabinovich GA, Gruppi A. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: functional cross-talk and implications during Trypanosoma cruzi infection. J. Immunol. 2004;172:493–502. - PubMed
-
- Barat-Houari M, Hilliou F, Jousset F-X, Sofer L, Deleury E, Rocher J, Ravallec M, Galibert L, Delobel P, Feyereisen R, Fournier P, Volkoff A-N. Gene expression profiling of Spodoptera frugiperda hemocytes and fat body using cDNA microarray reveals polydnavirus-associated variations in lepidoperan host gene transcript levels. BMC Genomics. 2006;7:160–179. - PMC - PubMed
-
- Barondes SH, Cooper DNW, Gitt MA, Leffler H. Galectins: Structure and function of a large family of animal lectins. J. Biol. Chem. 1994;269:20807–20810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

