32 wk old C3H/HeJ mice actively respond to mechanical loading
- PMID: 18280231
- PMCID: PMC2366046
- DOI: 10.1016/j.bone.2007.12.222
32 wk old C3H/HeJ mice actively respond to mechanical loading
Abstract
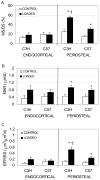
Numerous studies indicate that C3H/HeJ (C3H) mice are mildly responsive to mechanical loading compared to C57BL/6J (C57) mice. Guided by data indicating high baseline periosteal osteoblast activity in 16 wk C3H mice, we speculated that simply allowing the C3H mice to age until basal periosteal bone formation was equivalent to that of 16 wk C57 mice would restore mechanoresponsiveness in C3H mice. We tested this hypothesis by subjecting the right tibiae of 32 wk old C3H mice and 16 wk old C57 mice to low magnitude rest-inserted loading (peak strain: 1235 mu epsilon) and then exposing the right tibiae of 32 wk C3H mice to low (1085 mu epsilon) or moderate (1875 mu epsilon) magnitude cyclic loading. The osteoblastic response to loading on the endocortical and periosteal surfaces was evaluated via dynamic histomorphometry. At 32 wk of age, C3H mice responded to low magnitude rest-inserted loading with significantly elevated periosteal mineralizing surface, mineral apposition rate and bone formation compared to unloaded contralateral bones. Surprisingly, the periosteal bone formation induced by low magnitude rest-inserted loading in C3H mice exceeded that induced in 16 wk C57 mice. At 32 wk of age, C3H mice also demonstrated an elevated response to increased magnitudes of cyclic loading. We conclude that a high level of basal osteoblast function in 16 wk C3H mice appears to overwhelm the ability of the tissue to respond to an otherwise anabolic mechanical loading stimulus. However, when basal surface osteoblast activity is equivalent to that of 16 wk C57 mice, C3H mice demonstrate a clear ability to respond to either rest-inserted or cyclic loading.
Figures


Similar articles
-
Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J mice but not in C3H/Hej mice.Calcif Tissue Int. 2000 Apr;66(4):298-306. doi: 10.1007/s002230010060. Calcif Tissue Int. 2000. PMID: 10742449
-
Bone response to in vivo mechanical loading in two breeds of mice.Calcif Tissue Int. 1998 Nov;63(5):442-9. doi: 10.1007/s002239900554. Calcif Tissue Int. 1998. PMID: 9799831
-
Rest-inserted loading rapidly amplifies the response of bone to small increases in strain and load cycles.J Appl Physiol (1985). 2007 May;102(5):1945-52. doi: 10.1152/japplphysiol.00507.2006. Epub 2007 Jan 25. J Appl Physiol (1985). 2007. PMID: 17255366
-
Mice lacking thrombospondin 2 show an atypical pattern of endocortical and periosteal bone formation in response to mechanical loading.Bone. 2006 Mar;38(3):310-6. doi: 10.1016/j.bone.2005.08.027. Epub 2005 Nov 14. Bone. 2006. PMID: 16290255
-
Enabling bone formation in the aged skeleton via rest-inserted mechanical loading.Bone. 2003 Dec;33(6):946-55. doi: 10.1016/j.bone.2003.07.009. Bone. 2003. PMID: 14678854
Cited by
-
Bone strength and composition in spacefaring rodents: systematic review and meta-analysis.NPJ Microgravity. 2022 Apr 13;8(1):10. doi: 10.1038/s41526-022-00195-7. NPJ Microgravity. 2022. PMID: 35418128 Free PMC article.
-
Experimental and finite element analysis of dynamic loading of the mouse forearm.J Orthop Res. 2014 Dec;32(12):1580-8. doi: 10.1002/jor.22720. Epub 2014 Sep 5. J Orthop Res. 2014. PMID: 25196694 Free PMC article.
-
Increased deformations are dispensable for encapsulated cell mechanoresponse in engineered bone analogs mimicking aging bone marrow.Mechanobiol Med. 2025 Mar;3(1):100097. doi: 10.1016/j.mbm.2024.100097. Epub 2024 Oct 1. Mechanobiol Med. 2025. PMID: 40134991 Free PMC article.
-
Separating Fluid Shear Stress from Acceleration during Vibrations in Vitro: Identification of Mechanical Signals Modulating the Cellular Response.Cell Mol Bioeng. 2012 Sep 1;5(3):266-276. doi: 10.1007/s12195-012-0231-1. Epub 2012 May 9. Cell Mol Bioeng. 2012. PMID: 23074384 Free PMC article.
-
Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones.Bone. 2010 Feb;46(2):314-21. doi: 10.1016/j.bone.2009.08.054. Epub 2009 Sep 3. Bone. 2010. PMID: 19733269 Free PMC article.
References
-
- Eisman JA, Sambrook PN, Kelly PJ, Pocock NA. Exercise and its interaction with genetic influences in the determination of bone mineral density. Am J Med. 1991;91(5B):5S–9S. - PubMed
-
- Krall EA, Dawson-Hughes B. Heritable and life-style determinants of bone mineral density. J Bone Miner Res. 1993;8(1):1–9. - PubMed
-
- Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Bone mass, lean mass, and fat mass: same genes or same environments? Am J Epidemiol. 1998;147(1):3–16. - PubMed
-
- Itoh S, Udagawa N, Takahashi N, Yoshitake F, Narita H, Ebisu S, Ishihara K. A critical role for interleukin-6 family-mediated Stat3 activation in osteoblast differentiation and bone formation. Bone. 2006;39(3):505–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

