Ran-binding protein 3 phosphorylation links the Ras and PI3-kinase pathways to nucleocytoplasmic transport
- PMID: 18280241
- PMCID: PMC2266693
- DOI: 10.1016/j.molcel.2007.12.024
Ran-binding protein 3 phosphorylation links the Ras and PI3-kinase pathways to nucleocytoplasmic transport
Abstract
The major participants of the Ras/ERK and PI3-kinase (PI3K) pathways are well characterized. The cellular response to activation of these pathways, however, can vary dramatically. How differences in signal strength, timing, spatial location, and cellular context promote specific cell-fate decisions remains unclear. Nuclear transport processes can have a major impact on the determination of cell fate; however, little is known regarding how nuclear transport is regulated by or regulates these pathways. Here we show that RSK and Akt, which are activated downstream of Ras/ERK and PI3K, respectively, modulate the Ran gradient and nuclear transport by interacting with, phosphorylating, and regulating Ran-binding protein 3 (RanBP3) function. Our findings highlight an important link between two major cell-fate determinants: nuclear transport and the Ras/ERK/RSK and PI3K/Akt signaling pathways.
Figures
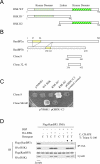
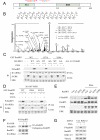
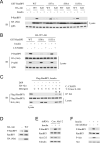

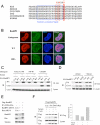

References
-
- Anjum R, Roux PP, Ballif BA, Gygi SP, Blenis J. The tumor suppressor DAP kinase is a target of RSK-mediated survival signaling. Curr Biol. 2005;15:1762–1767. - PubMed
-
- Arnaoutov A, Azuma Y, Ribbeck K, Joseph J, Boyarchuk Y, Karpova T, McNally J, Dasso M. Crm1 is a mitotic effector of Ran-GTP in somatic cells. Nat Cell Biol. 2005;7:626–632. - PubMed
-
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. - PubMed
-
- Clarke PR. The Crm de la creme of mitosis. Nat Cell Biol. 2005;7:551–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

