Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest
- PMID: 18280465
- PMCID: PMC2755085
- DOI: 10.1016/j.ydbio.2008.01.018
Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest
Abstract
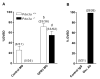
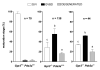
Although it is established that cAMP accumulation plays a pivotal role in preventing meiotic resumption in mammalian oocytes, the mechanisms controlling cAMP levels in the female gamete have remained elusive. Both production of cAMP via GPCRs/Gs/adenylyl cyclases endogenous to the oocyte as well as diffusion from the somatic compartment through gap junctions have been implicated in maintaining cAMP at levels that preclude maturation. Here we have used a genetic approach to investigate the different biochemical pathways contributing to cAMP accumulation and maturation in mouse oocytes. Because cAMP hydrolysis is greatly decreased and cAMP accumulates above a threshold, oocytes deficient in PDE3A do not resume meiosis in vitro or in vivo, resulting in complete female infertility. In vitro, inactivation of Gs or downregulation of the GPCR GPR3 causes meiotic resumption in the Pde3a null oocytes. Crossing of Pde3a(-/-) mice with Gpr3(-/-) mice causes partial recovery of female fertility. Unlike the complete meiotic block of the Pde3a null mice, oocyte maturation is restored in the double knockout, although it occurs prematurely as described for the Gpr3(-/-) mouse. The increase in cAMP that follows PDE3A ablation is not detected in double mutant oocytes, confirming that GPR3 functions upstream of PDE3A in the regulation of oocyte cAMP. Metabolic coupling between oocytes and granulosa cells was not affected in follicles from the single or double mutant mice, suggesting that diffusion of cAMP is not prevented. Finally, simultaneous ablation of GPR12, an additional receptor expressed in the oocyte, does not modify the Gpr3(-/-) phenotype. Taken together, these findings demonstrate that Gpr3 is epistatic to Pde3a and that fertility as well as meiotic arrest in the PDE3A-deficient oocyte is dependent on the activity of GPR3. These findings also suggest that cAMP diffusion through gap junctions or the activity of additional receptors is not sufficient by itself to maintain the meiotic arrest in the mouse oocyte.
Figures




References
-
- Aberdam E, Hanski E, Dekel N. Maintenance of meiotic arrest in isolated rat oocytes by the invasive adenylate cyclase of Bordetella pertussis. Biol Reprod. 1987;36:530–5. - PubMed
-
- Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol. 2001;233:258–70. - PubMed
-
- Bornslaeger EA, Schultz RM. Regulation of mouse oocyte maturation: effect of elevating cumulus cell cAMP on oocyte cAMP levels. Biol Reprod. 1985;33:698–704. - PubMed
-
- Chesnel F, Wigglesworth K, Eppig JJ. Acquisition of meiotic competence by denuded mouse oocytes: participation of somatic-cell product(s) and cAMP. Dev Biol. 1994;161:285–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

