A comprehensive approach toward novel serum biomarkers for benign prostatic hyperplasia: the MPSA Consortium
- PMID: 18280515
- PMCID: PMC3105378
- DOI: 10.1016/j.juro.2007.11.049
A comprehensive approach toward novel serum biomarkers for benign prostatic hyperplasia: the MPSA Consortium
Abstract
Purpose: Clinical benign prostatic hyperplasia is primarily diagnosed based on a diverse array of progressive lower urinary tract symptoms and is likely distinct from histological benign prostatic hyperplasia, which is detected by the presence of nonmalignant proliferation of prostate cells but may or may not be associated with symptoms. Pharmacological management of lower urinary tract symptoms has emerged as an effective initial treatment for clinical benign prostatic hyperplasia due to the introduction of new drug therapies shown to be effective in recent large clinical trials. Despite advances in symptom management and research into disease pathology, diagnostic strategies for the prediction of benign prostatic hyperplasia progression and response to drug modalities are lacking, and questions remain as to the molecular differences underlying clinical (symptomatic) vs histological (nonsymptomatic) benign prostatic hyperplasia.
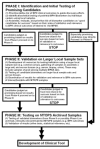
Materials and methods: As part of the Medical Therapy of Prostatic Symptoms (MTOPS) clinical trial, which demonstrated the effectiveness of combination drug therapy in slowing benign prostatic hyperplasia progression, an archive of biological specimens linked to clinical data was collected for future profiling of disease pathology and changes associated with response to drug therapy. The MTOPS Prostatic Samples Analysis (MPSA) Consortium was established to identify and validate molecular markers that may better define benign prostatic hyperplasia related pathologies, identify risk of progression of lower urinary tract symptoms, and predict response to drug therapy using the MTOPS archive. The cooperating MPSA Biomarker Discovery Sites and Pathology Coordinating Center use diverse methodologies and scientific approaches as well as unique expertise to address the goals of the Consortium.
Results: To date the MPSA has identified a number of promising biomarkers as well as other molecular and cellular changes associated with benign prostatic hyperplasia.
Conclusions: These findings and ongoing Consortium discovery efforts have the potential to provide a greater understanding of the defects underlying disease pathology, and may lead to the development of early and more effective pharmacological treatment strategies for benign prostatic hyperplasia.
Figures





References
-
- Roehrborn CG, McConnell JD, Saltzman B, Bergner D, Gray T, Narayan P, et al. Storage (irritative) and voiding (obstructive) symptoms as predictors of benign prostatic hyperplasia progression and related outcomes. Eur Urol. 2002;42:1. - PubMed
-
- McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12:S122. - PubMed
-
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America Project: benign prostatic hyperplasia. J Urol. 2005;173:1256. - PubMed
-
- Sarma AV, Jacobson DJ, McGree ME, Roberts RO, Lieber M, Jacobsen SJ. A population based study of incidence and treatment of benign prostatic hyperplasia among residents of Olmsted County, Minnesota: 1987 to 1997. J Urol. 2003;173:2048. - PubMed
-
- Chapple CR. Pharmacological therapy of benign prostatic hyperplasia/lower urinary tract symptoms: an overview for the practising clinician. BJU Int. 2004;94:738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK063661/DK/NIDDK NIH HHS/United States
- DK63594/DK/NIDDK NIH HHS/United States
- U01 DK063665/DK/NIDDK NIH HHS/United States
- DK63593/DK/NIDDK NIH HHS/United States
- DK63597/DK/NIDDK NIH HHS/United States
- DK63661/DK/NIDDK NIH HHS/United States
- DK63665/DK/NIDDK NIH HHS/United States
- AG022312/AG/NIA NIH HHS/United States
- U01 DK063597/DK/NIDDK NIH HHS/United States
- U01 DK063593/DK/NIDDK NIH HHS/United States
- U01 DK063594/DK/NIDDK NIH HHS/United States
- DK63587/DK/NIDDK NIH HHS/United States
- U01 DK063587/DK/NIDDK NIH HHS/United States
- U01 AG022312/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

