Ecstasy (3,4-methylenedioxymethamphetamine) limits murine gammaherpesvirus-68 induced monokine expression
- PMID: 18280699
- PMCID: PMC4275657
- DOI: 10.1016/j.bbi.2008.01.002
Ecstasy (3,4-methylenedioxymethamphetamine) limits murine gammaherpesvirus-68 induced monokine expression
Abstract
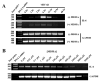
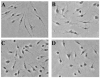
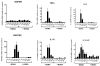
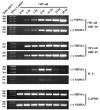
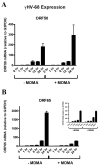
While Ecstasy (3,4-methylenedioxymethamphetamine, MDMA) has been shown to modulate immune responses, no studies have addressed drug-induced alterations to viral infection. In this study, bone marrow-derived macrophages were exposed to MDMA, then infected with murine gammaherpesvirus-68, and the expression of monokines assessed. MDMA-induced reductions in virus-stimulated monokine mRNA expression were observed in a dose-dependent manner. In particular, IL-6 mRNA expression and secretion was significantly decreased in gammaherpesvirus-infected macrophages exposed to MDMA. Concentrations of MDMA capable of reducing monokine production did not induce significant cell death and allowed normal viral gene expression. These studies represent the first to demonstrate the ability of this drug of abuse to alter a viral-induced macrophage response.
Figures



Similar articles
-
Methylenedioxymethamphetamine ('Ecstasy')-induced immunosuppression: a cause for concern?Br J Pharmacol. 2010 Sep;161(1):17-32. doi: 10.1111/j.1476-5381.2010.00899.x. Br J Pharmacol. 2010. PMID: 20718737 Free PMC article. Review.
-
3,4-Methylenedioxymethamphetamine (MDMA) alters acute gammaherpesvirus burden and limits interleukin 27 responses in a mouse model of viral infection.Drug Alcohol Depend. 2011 Jul 1;116(1-3):211-21. doi: 10.1016/j.drugalcdep.2010.12.019. Epub 2011 Jan 26. Drug Alcohol Depend. 2011. PMID: 21269783 Free PMC article.
-
Selective modulation of immune function resulting from in vitro exposure to methylenedioxymethamphetamine (Ecstasy).Toxicology. 1995 Jan 19;96(1):59-69. doi: 10.1016/0300-483x(94)02955-t. Toxicology. 1995. PMID: 7863512
-
Macrolide antibiotics promote the LPS-induced upregulation of prostaglandin E receptor EP2 and thus attenuate macrolide suppression of IL-6 production.Prostaglandins Leukot Essent Fatty Acids. 2007 Mar;76(3):181-8. doi: 10.1016/j.plefa.2006.12.005. Epub 2007 Feb 26. Prostaglandins Leukot Essent Fatty Acids. 2007. PMID: 17324565
-
Methylenedioxymethamphetamine (MDMA, 'Ecstasy'): a stressor on the immune system.Immunology. 2004 Apr;111(4):357-67. doi: 10.1111/j.0019-2805.2004.01847.x. Immunology. 2004. PMID: 15056370 Free PMC article. Review.
Cited by
-
Methylenedioxymethamphetamine ('Ecstasy')-induced immunosuppression: a cause for concern?Br J Pharmacol. 2010 Sep;161(1):17-32. doi: 10.1111/j.1476-5381.2010.00899.x. Br J Pharmacol. 2010. PMID: 20718737 Free PMC article. Review.
-
Psychedelics and Immunomodulation: Novel Approaches and Therapeutic Opportunities.Front Immunol. 2015 Jul 14;6:358. doi: 10.3389/fimmu.2015.00358. eCollection 2015. Front Immunol. 2015. PMID: 26236313 Free PMC article. Review.
-
3,4-methylenedioxymethamphetamine (MDMA--Ecstasy) decreases neutrophil activity through the glucocorticoid pathway and impairs host resistance to Listeria monocytogenes infection in mice.J Neuroimmune Pharmacol. 2014 Dec;9(5):690-702. doi: 10.1007/s11481-014-9562-0. Epub 2014 Aug 12. J Neuroimmune Pharmacol. 2014. PMID: 25113903
-
3,4-Methylenedioxymethamphetamine (MDMA) alters acute gammaherpesvirus burden and limits interleukin 27 responses in a mouse model of viral infection.Drug Alcohol Depend. 2011 Jul 1;116(1-3):211-21. doi: 10.1016/j.drugalcdep.2010.12.019. Epub 2011 Jan 26. Drug Alcohol Depend. 2011. PMID: 21269783 Free PMC article.
-
Murine gammaherpesvirus-68 expands, but does not activate, CD11b+ gr-1+ splenocytes in vivo.J Inflamm (Lond). 2012 Apr 16;9:14. doi: 10.1186/1476-9255-9-14. J Inflamm (Lond). 2012. PMID: 22507226 Free PMC article.
References
-
- Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol. 1988;149:159–163. - PubMed
-
- Bowman CC, Bost KL. Cyclooxygenase-2-mediated prostaglandin E2 production in mesenteric lymph nodes and in cultured macrophages and dendritic cells after infection with Salmonella. J Immunol. 2004;172:2469–2475. - PubMed
-
- Boyle NT, Connor TJ. MDMA (“Ecstasy”) suppresses the innate IFN-gamma response in vivo: A critical role for the anti-inflammatory cytokine IL-10. Eur J Pharmacol. 2007;572:228–238. - PubMed
-
- Capela JP, Fernandes E, Remiao F, Bastos ML, Meisel A, Carvalho F. Ecstasy induces apoptosis via 5-HT(2A)-receptor stimulation in cortical neurons. Neurotoxicology. 2007;28:868–875. - PubMed
-
- Capela JP, Ruscher K, Lautenschlager M, Freyer D, Dirnagl U, Gaio AR, Bastos ML, Meisel A, Carvalho F. Ecstasy-induced cell death in cortical neuronal cultures is serotonin 2A-receptor-dependent and potentiated under hyperthermia. Neuroscience. 2006;139:1069–1081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

