Epigenetic mechanisms regulate Mallory Denk body formation in the livers of drug-primed mice
- PMID: 18281034
- PMCID: PMC2874464
- DOI: 10.1016/j.yexmp.2007.12.004
Epigenetic mechanisms regulate Mallory Denk body formation in the livers of drug-primed mice
Abstract
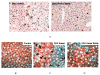
The mechanism of Mallory Denk body formation is still not fully understood, but growing evidence implicates epigenetic mechanisms in MDB formation. In a previous study the epigenetic memory of MDB formation remained intact for at least 4 months after withdrawal from the DDC diet. In the present study, mice were fed a diet containing DDC or a diet containing DDC and S-adenosylmethionine (SAMe) to investigate the epigenetic memory of MDB formation. DDC feeding caused an increase in histone 3 acetylation, a decrease in histone 3 trimethylation, and an increase in histone ubiquitinylation. The addition of SAMe to the DDC diet prevented the DDC induced decrease of H3K4 and H3K9 trimethylation and the increase in histone ubiquitinylation. Changes in histone modifying enzymes (HATs and HDACs), were also found in the liver nuclear extracts of the DDC/SAMe fed mice. Data mining of microarray analysis confirmed that gene expression changed with DDC refeeding, particularly the SAMe metabolizing enzymes, Mat2a, AMD, AHCY and Mthfr. SAMe supplementation prevented the decrease of AHCY and GNMT, and prevented the increase in Mthfr, which provides a mechanism to explain how DDC inhibits methylation of histones. The results indicate that SAMe prevented the epigenetic cellular memory involved in the MDB formation.
Figures

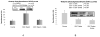
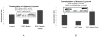




References
-
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the “magnificent seven”, function, metabolism and longevity. Ann Med. 2007;39(5):335–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

