The tec family tyrosine kinase Btk Regulates RANKL-induced osteoclast maturation
- PMID: 18281276
- PMCID: PMC2431049
- DOI: 10.1074/jbc.M708935200
The tec family tyrosine kinase Btk Regulates RANKL-induced osteoclast maturation
Abstract
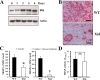
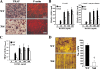
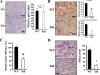
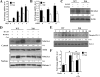
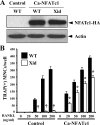
A spontaneous mutation in Bruton's tyrosine kinase (Btk) induces a defect in B-cell development that results in the immunodeficiency diseases X-linked agammaglobulinemia in humans and X-linked immunodeficiency (Xid) in mice. Here we show an unexpected role of Btk in osteoclast formation. When bone marrow cells derived from Xid mice were stimulated with receptor activator of NF-kappaB ligand, an osteoclast differentiation factor, they did not completely differentiate into mature multinucleated osteoclasts. Moreover, we found that the defects appeared to occur at the stage in which mononuclear preosteoclasts fuse to generate multinucleated cells. Supporting this notion, macrophages from Xid mice also failed to form multinucleated foreign body giant cells. The fusion defect of the Xid mutant osteoclasts was caused by decreased expression of nuclear factor of activated T cells c1 (NFATc1), a master regulator of osteoclast differentiation, as well as reduced expression of various osteoclast fusion-related molecules, such as the d2 isoform of vacuolar H(+)-ATPase V0 domain and the dendritic cell-specific transmembrane protein. This deficiency was completely rescued by the introduction of a constitutively active form of NFATc1 into bone marrow-derived macrophages. Our data provide strong evidence that Btk plays a critical role in osteoclast multinucleation by modulating the activity of NFATc1.
Figures
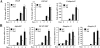

References
-
- Walsh, M. C., Kim, N., Kadono, Y., Rho, J., Lee, S. Y., Lorenzo, J., and Choi, Y. (2006) Annu. Rev. Immunol. 24 33-63 - PubMed
-
- Teitelbaum, S. L. (2000) Science 289 1504-1508 - PubMed
-
- Boyle, W. J., Simonet, W. S., and Lacey, D. L. (2003) Nature 423 337-342 - PubMed
-
- Lee, S. H., Rho, J., Jeong, D., Sul, J. Y., Kim, T., Kim, N., Kang, J. S., Miyamoto, T., Suda, T., Lee, S. K., Pignolo, R. J., Koczon-Jaremko, B., Lorenzo, J., and Choi, Y. (2006) Nat. Med. 12 1403-1409 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

