Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss
- PMID: 18281279
- PMCID: PMC2447638
- DOI: 10.1074/jbc.M707659200
Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss
Abstract
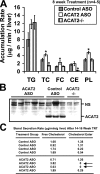
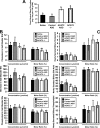
Deletion of acyl-CoA:cholesterol O-acyltransferase 2 (ACAT2) in mice results in resistance to diet-induced hypercholesterolemia and protection against atherosclerosis. Recently, our group has shown that liver-specific inhibition of ACAT2 via antisense oligonucleotide (ASO)-mediated targeting likewise limits atherosclerosis. However, whether this atheroprotective effect was mediated by: 1) prevention of packaging of cholesterol into apoB-containing lipoproteins, 2) augmentation of nascent HDL cholesterol secretion, or 3) increased hepatobiliary sterol secretion was not examined. Therefore, the purpose of these studies was to determine whether hepatic ACAT2 is rate-limiting in all three of these important routes of cholesterol homeostasis. Liver-specific depletion of ACAT2 resulted in reduced packaging of cholesterol into apoB-containing lipoproteins (very low density lipoprotein, intermediate density lipoprotein, and low density lipoprotein), whereas high density lipoprotein cholesterol levels remained unchanged. In the liver of ACAT2 ASO-treated mice, cholesterol ester accumulation was dramatically reduced, yet there was no reciprocal accumulation of unesterified cholesterol. Paradoxically, ASO-mediated depletion of hepatic ACAT2 promoted fecal neutral sterol excretion without altering biliary sterol secretion. Interestingly, during isolated liver perfusion, ACAT2 ASO-treated livers had augmented secretion rates of unesterified cholesterol and phospholipid. Furthermore, we demonstrate that liver-derived cholesterol from ACAT2 ASO-treated mice is preferentially delivered to the proximal small intestine as a precursor to fecal excretion. Collectively, these studies provide the first insight into the hepatic itinerary of cholesterol when cholesterol esterification is inhibited only in the liver, and provide evidence for a novel non-biliary route of fecal sterol loss.
Figures






Comment in
-
A new twist in the cholesterol metabolism-based enterohepatic connection.Gastroenterology. 2008 Aug;135(2):705-6. doi: 10.1053/j.gastro.2008.06.067. Epub 2008 Jul 9. Gastroenterology. 2008. PMID: 18619455 No abstract available.
Similar articles
-
Liver-specific inhibition of acyl-coenzyme a:cholesterol acyltransferase 2 with antisense oligonucleotides limits atherosclerosis development in apolipoprotein B100-only low-density lipoprotein receptor-/- mice.Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1814-20. doi: 10.1161/01.ATV.0000225289.30767.06. Epub 2006 May 4. Arterioscler Thromb Vasc Biol. 2006. PMID: 16675724
-
Inhibition of acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2) prevents dietary cholesterol-associated steatosis by enhancing hepatic triglyceride mobilization.J Biol Chem. 2010 May 7;285(19):14267-74. doi: 10.1074/jbc.M110.118422. Epub 2010 Mar 15. J Biol Chem. 2010. PMID: 20231283 Free PMC article.
-
Tissue-specific knockouts of ACAT2 reveal that intestinal depletion is sufficient to prevent diet-induced cholesterol accumulation in the liver and blood.J Lipid Res. 2012 Jun;53(6):1144-52. doi: 10.1194/jlr.M024356. Epub 2012 Mar 29. J Lipid Res. 2012. PMID: 22460046 Free PMC article.
-
Acyl coenzyme A: cholesterol acyltransferase inhibition and hepatic apolipoprotein B secretion.Clin Chim Acta. 1999 Aug;286(1-2):231-42. doi: 10.1016/s0009-8981(99)00104-7. Clin Chim Acta. 1999. PMID: 10511295 Review.
-
Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis.Curr Opin Lipidol. 2001 Apr;12(2):121-7. doi: 10.1097/00041433-200104000-00005. Curr Opin Lipidol. 2001. PMID: 11264983 Review.
Cited by
-
Regulation of reverse cholesterol transport - a comprehensive appraisal of available animal studies.Nutr Metab (Lond). 2012 Mar 29;9(1):25. doi: 10.1186/1743-7075-9-25. Nutr Metab (Lond). 2012. PMID: 22458435 Free PMC article.
-
Obesity-linked suppression of membrane-bound O-acyltransferase 7 (MBOAT7) drives non-alcoholic fatty liver disease.Elife. 2019 Oct 17;8:e49882. doi: 10.7554/eLife.49882. Elife. 2019. PMID: 31621579 Free PMC article.
-
Protein mediators of sterol transport across intestinal brush border membrane.Subcell Biochem. 2010;51:337-80. doi: 10.1007/978-90-481-8622-8_12. Subcell Biochem. 2010. PMID: 20213550 Free PMC article. Review.
-
Targeted Knockdown of Hepatic SOAT2 With Antisense Oligonucleotides Stabilizes Atherosclerotic Plaque in ApoB100-only LDLr-/- Mice.Arterioscler Thromb Vasc Biol. 2015 Sep;35(9):1920-7. doi: 10.1161/ATVBAHA.115.305747. Epub 2015 Jul 30. Arterioscler Thromb Vasc Biol. 2015. PMID: 26229140 Free PMC article.
-
The thyroid receptor modulator KB3495 reduces atherosclerosis independently of total cholesterol in the circulation in ApoE deficient mice.PLoS One. 2013 Dec 4;8(12):e78534. doi: 10.1371/journal.pone.0078534. eCollection 2013. PLoS One. 2013. PMID: 24324578 Free PMC article.
References
-
- Dietschy, J. M., and Turley, S. D. (2002) J. Biol. Chem. 277 3801-3804 - PubMed
-
- Dietschy, J. M., Turley, S. D., and Spady, D. K. (1993) J. Lipid Res. 34 1637-1659 - PubMed
-
- Brown, M. S., Kovanen, P. T., and Goldstein, J. L. (1981) Science 212 628-635 - PubMed
-
- Turley, S. D., and Dietshcy, J. M. (2003) Curr. Opin. Lipidol. 14 233-240 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

