Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia
- PMID: 18281291
- PMCID: PMC2447655
- DOI: 10.1074/jbc.M800102200
Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia
Retraction in
-
Withdrawal: Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia.J Biol Chem. 2023 Aug;299(8):105125. doi: 10.1016/j.jbc.2023.105125. Epub 2023 Aug 7. J Biol Chem. 2023. PMID: 37556879 Free PMC article. No abstract available.
Abstract
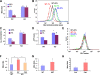
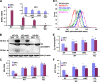
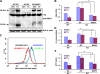
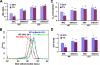
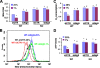
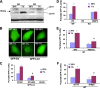
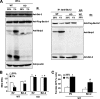
Autophagy is a process by which cytoplasmic organelles can be catabolized either to remove defective structures or as a means of providing macromolecules for energy generation under conditions of nutrient starvation. In this study we demonstrate that mitochondrial autophagy is induced by hypoxia, that this process requires the hypoxia-dependent factor-1-dependent expression of BNIP3 and the constitutive expression of Beclin-1 and Atg5, and that in cells subjected to prolonged hypoxia, mitochondrial autophagy is an adaptive metabolic response which is necessary to prevent increased levels of reactive oxygen species and cell death.
Figures

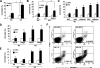

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

