The pathways and outcomes of mycobacterial NHEJ depend on the structure of the broken DNA ends
- PMID: 18281464
- PMCID: PMC2238672
- DOI: 10.1101/gad.1631908
The pathways and outcomes of mycobacterial NHEJ depend on the structure of the broken DNA ends
Abstract
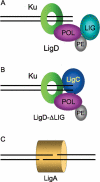
Mycobacteria can repair DNA double-strand breaks (DSBs) via a nonhomologous end-joining (NHEJ) system that includes a dedicated DNA ligase (LigD) and the DNA end-binding protein Ku. Here we exploit an improved plasmid-based NHEJ assay and a collection of Mycobacterium smegmatis strains bearing deletions or mutations in Ku or the DNA ligases to interrogate the contributions of LigD's three catalytic activities (polymerase, ligase, and 3' phosphoesterase) and structural domains (POL, LIG, and PE) to the efficiency and molecular outcomes of NHEJ in vivo. By analyzing in parallel the repair of blunt, 5' overhang, and 3' overhang DSBs, we discovered a novel end-joining pathway specific to breaks with 3' overhangs that is Ku- and LigD-independent and perfectly faithful. This 3' overhang NHEJ pathway is independent of ligases B and C; we surmise that it relies on NAD(+)-dependent LigA, the essential replicative ligase. We find that efficient repair of blunt and 5' overhang DSBs depends stringently on Ku and the LigD POL domain, but not on the LigD polymerase activity, which mainly serves to promote NHEJ infidelity. The lack of an effect of PE-inactivating LigD mutations on NHEJ outcomes, especially the balance between deletions and insertions at blunt or 5' overhang breaks, argues against LigD being the catalyst of deletion formation. Ligase-inactivating LigD mutations (or deletion of the LIG domain) have a modest impact on the efficiency of blunt and 5' overhang DSB repair, because the strand sealing activity can be provided in trans by one of the other resident ATP-dependent ligases (likely LigC). Reliance on the backup ligase is accompanied by a drastic loss of fidelity during blunt end and 5' overhang DSB repair. We conclude that the mechanisms of mycobacterial NHEJ are many and the outcomes depend on the initial structures of the DSBs and the available ensemble of end-processing and end-sealing components, which are not limited to Ku and LigD.
Figures




Comment in
-
Mechanistic flexibility as a conserved theme across 3 billion years of nonhomologous DNA end-joining.Genes Dev. 2008 Feb 15;22(4):411-5. doi: 10.1101/gad.1646608. Genes Dev. 2008. PMID: 18281457 Free PMC article. Review. No abstract available.
References
-
- Akey D., Martins A., Aniukwu J., Glickman M.S., Shuman S., Berger J.M. Crystal structure and nonhomologous end joining function of the ligase domain of Mycobacterium DNA ligase D. J. Biol. Chem. 2006;281:13412–13423. - PubMed
-
- Brissett N.C., Pitcher R.S., Juarez R., Picher A.J., Green A.J., Dafforn T.R., Fox G.C., Blanco L., Doherty A.J. Structure of a NHEJ polymerase-mediated synaptic complex. Science. 2007;318:456–459. - PubMed
-
- Della M., Palmbos P.L., Tseng H.M., Tonkin L.M., Daley J.M., Topper L.M., Pitcher R.S., Tomkinson A.E., Wilson T.E., Doherty A.J. Mycobacterial Ku and ligase proteins constitute a two-component NHEJ repair machine. Science. 2004;306:683–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
