Growth suppression of lung cancer cells by targeting cyclic AMP response element-binding protein
- PMID: 18281471
- PMCID: PMC2921320
- DOI: 10.1158/0008-5472.CAN-06-0249
Growth suppression of lung cancer cells by targeting cyclic AMP response element-binding protein
Abstract
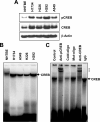
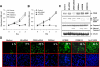
Genes regulated by cyclic AMP-response element-binding protein (CREB) have been reported to suppress apoptosis, induce cell proliferation, and mediate inflammation and tumor metastasis. However, it is not clear whether CREB is critically involved in lung carcinogenesis. We found that non-small cell lung cancer (NSCLC) cell lines exhibited elevated constitutive activity in CREB, in its immediate upstream kinases (ribosomal s6 kinase and extracellular signal kinase), and in the CREB-regulated cell survival proteins Bcl-2 and Bcl-xL. We hypothesized that constitutively active CREB is important to lung cancer cell growth and survival and therefore could be a potential therapeutic target for NSCLC. Ectopic expression of dominant repressor CREB and transfection with small interfering RNA against CREB suppressed the growth and survival of NSCLC cells and induced apoptotic cell death. Furthermore, treating H1734 NSCLC cells with an inhibitor of the CREB signaling pathway Ro-31-8220 inhibited CREB activation by blocking the activity of extracellular signal kinase and ribosomal s6 kinase, arrested the cell cycle at the G(2)-M phase, and subsequently induced apoptosis with the suppression of Bcl-2 and Bcl-xL expression. Ro-31-8220 suppressed both the anchorage-dependent and independent growth of NSCLC cells, but its cytotoxic effect was much less prominent in normal bronchial epithelial cells. Our results indicate that active CREB plays an important role in NSCLC cell growth and survival. Thus, agents that suppress CREB activation could have potential therapeutic value for NSCLC treatment.
Figures




References
-
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. - PubMed
-
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. - PubMed
-
- Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–95. - PubMed
-
- Conkright MD, Montminy M. CREB: the unindicted cancer co-conspirator. Trends Cell Biol. 2005;15:457–9. - PubMed
-
- Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science (New York, NY. 1999;286:2358–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

