Mice lacking the extracellular matrix protein MAGP1 display delayed thrombotic occlusion following vessel injury
- PMID: 18281502
- PMCID: PMC2288724
- DOI: 10.1182/blood-2007-07-101733
Mice lacking the extracellular matrix protein MAGP1 display delayed thrombotic occlusion following vessel injury
Abstract
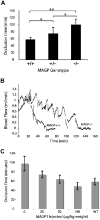
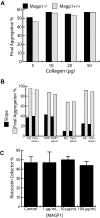
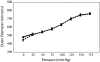
Mice lacking the extracellular matrix protein microfibril-associated glycoprotein-1 (MAGP1) display delayed thrombotic occlusion of the carotid artery following injury as well as prolonged bleeding from a tail vein incision. Normal occlusion times were restored when recombinant MAGP1 was infused into deficient animals prior to vessel wounding. Blood coagulation was normal in these animals as assessed by activated partial thromboplastin time and prothrombin time. Platelet number was lower in MAGP1-deficient mice, but the platelets showed normal aggregation properties in response to various agonists. MAGP1 was not found in normal platelets or in the plasma of wild-type mice. In ligand blot assays, MAGP1 bound to fibronectin, fibrinogen, and von Willebrand factor, but von Willebrand factor was the only protein of the 3 that bound to MAGP1 in surface plasmon resonance studies. These findings show that MAGP1, a component of microfibrils and vascular elastic fibers, plays a role in hemostasis and thrombosis.
Figures




Similar articles
-
The extracellular matrix protein MAGP1 supports thermogenesis and protects against obesity and diabetes through regulation of TGF-β.Diabetes. 2014 Jun;63(6):1920-32. doi: 10.2337/db13-1604. Epub 2014 Jan 23. Diabetes. 2014. PMID: 24458361 Free PMC article.
-
Losartan and captopril treatment rescue normal thrombus formation in microfibril associated glycoprotein-1 (MAGP1) deficient mice.Thromb Res. 2016 Feb;138:7-15. doi: 10.1016/j.thromres.2015.12.004. Epub 2015 Dec 10. Thromb Res. 2016. PMID: 26826502
-
Microfibril-associated glycoprotein 2 (MAGP2) loss of function has pleiotropic effects in vivo.J Biol Chem. 2013 Oct 4;288(40):28869-80. doi: 10.1074/jbc.M113.497727. Epub 2013 Aug 20. J Biol Chem. 2013. PMID: 23963447 Free PMC article.
-
Transport physics and biorheology in the setting of hemostasis and thrombosis.J Thromb Haemost. 2016 May;14(5):906-17. doi: 10.1111/jth.13280. Epub 2016 Mar 30. J Thromb Haemost. 2016. PMID: 26848552 Free PMC article. Review.
-
Fibronectin maintains the balance between hemostasis and thrombosis.Cell Mol Life Sci. 2016 Sep;73(17):3265-77. doi: 10.1007/s00018-016-2225-y. Epub 2016 Apr 21. Cell Mol Life Sci. 2016. PMID: 27098513 Free PMC article. Review.
Cited by
-
The extracellular matrix protein MAGP1 supports thermogenesis and protects against obesity and diabetes through regulation of TGF-β.Diabetes. 2014 Jun;63(6):1920-32. doi: 10.2337/db13-1604. Epub 2014 Jan 23. Diabetes. 2014. PMID: 24458361 Free PMC article.
-
The microfibril-associated glycoproteins (MAGPs) and the microfibrillar niche.Matrix Biol. 2015 Sep;47:13-33. doi: 10.1016/j.matbio.2015.05.003. Epub 2015 May 8. Matrix Biol. 2015. PMID: 25963142 Free PMC article. Review.
-
Investigation of age-related decline of microfibril-associated glycoprotein-1 in human skin through immunohistochemistry study.Clin Cosmet Investig Dermatol. 2013 Dec 6;6:317-23. doi: 10.2147/CCID.S51958. eCollection 2013. Clin Cosmet Investig Dermatol. 2013. PMID: 24353434 Free PMC article.
-
Oophorectomy-induced bone loss is attenuated in MAGP1-deficient mice.J Cell Biochem. 2012 Jan;113(1):93-9. doi: 10.1002/jcb.23331. J Cell Biochem. 2012. PMID: 21898536 Free PMC article.
-
EMILIN2 regulates platelet activation, thrombus formation, and clot retraction.PLoS One. 2015 Feb 6;10(2):e0115284. doi: 10.1371/journal.pone.0115284. eCollection 2015. PLoS One. 2015. PMID: 25658937 Free PMC article.
References
-
- Gibson MA, Cleary EG. The immunohistochemical localization of microfibril-associated glycoprotein (MAGP) in elastic and non-elastic tissues. Immunol Cell Biol. 1987;65:345–356. - PubMed
-
- Gibson MA, Kumaratilake JS, Cleary EG. The protein components of the 12-nanometer microfibrils of elastic and non-elastic tissues. J Biol Chem. 1989;264:4590–4598. - PubMed
-
- Kielty CM, Sherratt MJ, Marson A, Baldock C. Fibrillin microfibrils. Adv Protein Chem. 2005;70:405–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

