Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103
- PMID: 18281659
- PMCID: PMC3715075
- DOI: 10.1200/JCO.2007.14.3461
Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103
Abstract
Purpose: To assess the efficacy and toxicity of carboplatin, etoposide, and the bcl-2 antisense oligonucleotide oblimersen as initial therapy for extensive-stage small-cell lung cancer (ES-SCLC). bcl-2 has been implicated as a key factor in SCLC oncogenesis and chemotherapeutic resistance.
Patients and methods: A 3:1 randomized phase II study was performed to evaluate carboplatin and etoposide with (arm A) or without oblimersen (arm B) in 56 assessable patients with chemotherapy-naïve ES-SCLC. Outcome measures including toxicity, objective response rate, complete response rate, failure-free survival, overall survival, and 1-year survival rate.
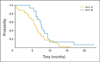
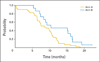
Results: Oblimersen was associated with slightly more grade 3 to 4 hematologic toxicity (88% v 60%; P = .05). Response rates were 61% (95% CI, 45% to 76%) for arm A and 60% (95% CI, 32% to 84%) for arm B. The percentage of patients alive at 1 year was 24% (95% CI, 12% to 40%) with oblimersen, and 47% (95% CI, 21% to 73%) without oblimersen. Hazard ratios for failure-free survival (1.79; P = .07) and overall survival (2.13; P = .02) suggested worse outcome for patients receiving oblimersen. These results hold when adjusted for other prognostic factors, such as weight loss, in multivariate regression analysis.
Conclusion: Despite extensive data supporting a critical role for Bcl-2 in chemoresistance in SCLC, addition of oblimersen to a standard regimen for this disease did not improve any clinical outcome measure. Emerging data from several groups suggest that this lack of efficacy may be due to insufficient suppression of Bcl-2 in vivo. Additional evaluation of this agent in SCLC is not warranted.
Conflict of interest statement
Figures
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Murray N, Turrisi AT., III A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. - PubMed
-
- Ikegaki N, Katsumata M, Minna J, et al. Expression of bcl-2 in small cell lung carcinoma cells. Cancer Res. 1994;54:6–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA11789/CA/NCI NIH HHS/United States
- CA35113/CA/NCI NIH HHS/United States
- CA41287/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- CA45389/CA/NCI NIH HHS/United States
- CA45808/CA/NCI NIH HHS/United States
- CA16450/CA/NCI NIH HHS/United States
- CA77440/CA/NCI NIH HHS/United States
- CA45564/CA/NCI NIH HHS/United States
- CA04326/CA/NCI NIH HHS/United States
- CA21060/CA/NCI NIH HHS/United States
- CA47642/CA/NCI NIH HHS/United States
- CA45418/CA/NCI NIH HHS/United States
- CA77298/CA/NCI NIH HHS/United States
- CA31983/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- CA33601/CA/NCI NIH HHS/United States
- CA77597/CA/NCI NIH HHS/United States
- CA77406/CA/NCI NIH HHS/United States
- CA47577/CA/NCI NIH HHS/United States
- CA32291/CA/NCI NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical