Stimulus design for model selection and validation in cell signaling
- PMID: 18282085
- PMCID: PMC2323406
- DOI: 10.1371/journal.pcbi.0040030
Stimulus design for model selection and validation in cell signaling
Abstract
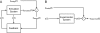
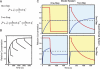
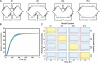
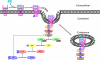
Mechanism-based chemical kinetic models are increasingly being used to describe biological signaling. Such models serve to encapsulate current understanding of pathways and to enable insight into complex biological processes. One challenge in model development is that, with limited experimental data, multiple models can be consistent with known mechanisms and existing data. Here, we address the problem of model ambiguity by providing a method for designing dynamic stimuli that, in stimulus-response experiments, distinguish among parameterized models with different topologies, i.e., reaction mechanisms, in which only some of the species can be measured. We develop the approach by presenting two formulations of a model-based controller that is used to design the dynamic stimulus. In both formulations, an input signal is designed for each candidate model and parameterization so as to drive the model outputs through a target trajectory. The quality of a model is then assessed by the ability of the corresponding controller, informed by that model, to drive the experimental system. We evaluated our method on models of antibody-ligand binding, mitogen-activated protein kinase (MAPK) phosphorylation and de-phosphorylation, and larger models of the epidermal growth factor receptor (EGFR) pathway. For each of these systems, the controller informed by the correct model is the most successful at designing a stimulus to produce the desired behavior. Using these stimuli we were able to distinguish between models with subtle mechanistic differences or where input and outputs were multiple reactions removed from the model differences. An advantage of this method of model discrimination is that it does not require novel reagents, or altered measurement techniques; the only change to the experiment is the time course of stimulation. Taken together, these results provide a strong basis for using designed input stimuli as a tool for the development of cell signaling models.
Conflict of interest statement
Figures

References
-
- Kitano H. Systems biology: A brief overview. Science. 2002;295:1662–1664. - PubMed
-
- Kremling A, Saez-Rodriguez J. Systems biology—An engineering perspective. J Biotechnol. 2007;129:329–351. - PubMed
-
- Lipschultz CA, Li Y, Smith-Gill S. Experimental design for analysis of complex kinetics using surface plasmon resonance. Methods. 2000;20:310–318. - PubMed
-
- Box GEP, Hill WJ. Discrimination among mechanistic models. Technometrics. 1967;9:57–71.
-
- Asprey SP, Macchietto S. Designing robust optimal dynamic experiments. J Process Contr. 2002;12:545–556.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

