A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease
- PMID: 18282093
- PMCID: PMC2242832
- DOI: 10.1371/journal.ppat.0040029
A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease
Abstract
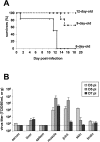
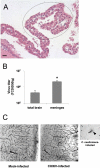
Chikungunya virus (CHIKV) is a re-emerging arbovirus responsible for a massive outbreak currently afflicting the Indian Ocean region and India. Infection from CHIKV typically induces a mild disease in humans, characterized by fever, myalgia, arthralgia, and rash. Cases of severe CHIKV infection involving the central nervous system (CNS) have recently been described in neonates as well as in adults with underlying conditions. The pathophysiology of CHIKV infection and the basis for disease severity are unknown. To address these critical issues, we have developed an animal model of CHIKV infection. We show here that whereas wild type (WT) adult mice are resistant to CHIKV infection, WT mouse neonates are susceptible and neonatal disease severity is age-dependent. Adult mice with a partially (IFN-alpha/betaR(+/-)) or totally (IFN-alpha/betaR(-/-)) abrogated type-I IFN pathway develop a mild or severe infection, respectively. In mice with a mild infection, after a burst of viral replication in the liver, CHIKV primarily targets muscle, joint, and skin fibroblasts, a cell and tissue tropism similar to that observed in biopsy samples of CHIKV-infected humans. In case of severe infections, CHIKV also disseminates to other tissues including the CNS, where it specifically targets the choroid plexuses and the leptomeninges. Together, these data indicate that CHIKV-associated symptoms match viral tissue and cell tropisms, and demonstrate that the fibroblast is a predominant target cell of CHIKV. These data also identify the neonatal phase and inefficient type-I IFN signaling as risk factors for severe CHIKV-associated disease. The development of a permissive small animal model will expedite the testing of future vaccines and therapeutic candidates.
Conflict of interest statement
Figures






References
-
- Mason PJ, Haddow AJ. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53; an additional note on Chikungunya virus isolations and serum antibodies. Trans R Soc Trop Med Hyg. 1957;51:238–240. - PubMed
-
- Enserink M. Infectious diseases. Massive outbreak draws fresh attention to little-known virus. Science. 2006;311:1085. - PubMed
-
- Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

