Human innate Mycobacterium tuberculosis-reactive alphabetaTCR+ thymocytes
- PMID: 18282101
- PMCID: PMC2242840
- DOI: 10.1371/journal.ppat.0040039
Human innate Mycobacterium tuberculosis-reactive alphabetaTCR+ thymocytes
Abstract
The control of Mycobacterium tuberculosis (Mtb) infection is heavily dependent on the adaptive Th1 cellular immune response. Paradoxically, optimal priming of the Th1 response requires activation of priming dendritic cells with Th1 cytokine IFN-gamma. At present, the innate cellular mechanisms required for the generation of an optimal Th1 T cell response remain poorly characterized. We hypothesized that innate Mtb-reactive T cells provide an early source of IFN-gamma to fully activate Mtb-exposed dendritic cells. Here, we report the identification of a novel population of Mtb-reactive CD4(-) alphabetaTCR(+) innate thymocytes. These cells are present at high frequencies, respond to Mtb-infected cells by producing IFN-gamma directly ex vivo, and display characteristics of effector memory T cells. This novel innate population of Mtb-reactive T cells will drive further investigation into the role of these cells in the containment of Mtb following infectious exposure. Furthermore, this is the first demonstration of a human innate pathogen-specific alphabetaTCR(+) T cell and is likely to inspire further investigation into innate T cells recognizing other important human pathogens.
Conflict of interest statement
Figures
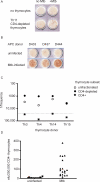



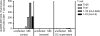
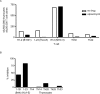

References
-
- Maher D, Raviglione M. Global epidemiology of tuberculosis. Clin Chest Med. 2005;26:167–182. - PubMed
-
- Verver S, Warren RM, Munch Z, Richardson M, van der Spuy GD, et al. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet. 2004;363:212–214. - PubMed
-
- Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb) 2004;84:93–101. - PubMed
-
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. - PubMed
-
- Bhatt K, Hickman SP, Salgame P. Cutting edge: A new approach to modeling early lung immunity in murine tuberculosis. J Immunol. 2004;172:2748–2751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

