Iron source preference and regulation of iron uptake in Cryptococcus neoformans
- PMID: 18282105
- PMCID: PMC2242830
- DOI: 10.1371/journal.ppat.0040045
Iron source preference and regulation of iron uptake in Cryptococcus neoformans
Abstract
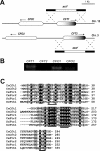
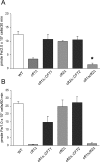
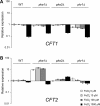
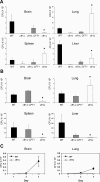
The level of available iron in the mammalian host is extremely low, and pathogenic microbes must compete with host proteins such as transferrin for iron. Iron regulation of gene expression, including genes encoding iron uptake functions and virulence factors, is critical for the pathogenesis of the fungus Cryptococcus neoformans. In this study, we characterized the roles of the CFT1 and CFT2 genes that encode C. neoformans orthologs of the Saccharomyces cerevisiae high-affinity iron permease FTR1. Deletion of CFT1 reduced growth and iron uptake with ferric chloride and holo-transferrin as the in vitro iron sources, and the cft1 mutant was attenuated for virulence in a mouse model of infection. A reduction in the fungal burden in the brains of mice infected with the cft1 mutant was observed, thus suggesting a requirement for reductive iron acquisition during cryptococcal meningitis. CFT2 played no apparent role in iron acquisition but did influence virulence. The expression of both CFT1 and CFT2 was influenced by cAMP-dependent protein kinase, and the iron-regulatory transcription factor Cir1 positively regulated CFT1 and negatively regulated CFT2. Overall, these results indicate that C. neoformans utilizes iron sources within the host (e.g., holo-transferrin) that require Cft1 and a reductive iron uptake system.
Conflict of interest statement
Figures
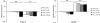

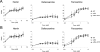



Similar articles
-
An encapsulation of iron homeostasis and virulence in Cryptococcus neoformans.Trends Microbiol. 2013 Sep;21(9):457-65. doi: 10.1016/j.tim.2013.05.007. Epub 2013 Jun 25. Trends Microbiol. 2013. PMID: 23810126 Free PMC article. Review.
-
A human fungal pathogen Cryptococcus neoformans expresses three distinct iron permease homologs.J Microbiol Biotechnol. 2012 Dec;22(12):1644-52. doi: 10.4014/jmb.1209.09019. J Microbiol Biotechnol. 2012. PMID: 23221526
-
Impact of iron chelators on growth and expression of iron-related genes of Cryptococcus species.J Mycol Med. 2020 Apr;30(1):100905. doi: 10.1016/j.mycmed.2019.100905. Epub 2019 Oct 11. J Mycol Med. 2020. PMID: 31706700
-
The Monothiol Glutaredoxin Grx4 Regulates Iron Homeostasis and Virulence in Cryptococcus neoformans.mBio. 2018 Dec 4;9(6):e02377-18. doi: 10.1128/mBio.02377-18. mBio. 2018. PMID: 30514787 Free PMC article.
-
Iron and fungal pathogenesis: a case study with Cryptococcus neoformans.Cell Microbiol. 2008 Feb;10(2):277-84. doi: 10.1111/j.1462-5822.2007.01077.x. Epub 2007 Nov 27. Cell Microbiol. 2008. PMID: 18042257 Review.
Cited by
-
Adaptation of Cryptococcus neoformans to mammalian hosts: integrated regulation of metabolism and virulence.Eukaryot Cell. 2012 Feb;11(2):109-18. doi: 10.1128/EC.05273-11. Epub 2011 Dec 2. Eukaryot Cell. 2012. PMID: 22140231 Free PMC article. Review.
-
Cryptococcus neoformans requires the ESCRT protein Vps23 for iron acquisition from heme, for capsule formation, and for virulence.Infect Immun. 2013 Jan;81(1):292-302. doi: 10.1128/IAI.01037-12. Epub 2012 Nov 6. Infect Immun. 2013. PMID: 23132495 Free PMC article.
-
Influence of iron regulation on the metabolome of Cryptococcus neoformans.PLoS One. 2012;7(7):e41654. doi: 10.1371/journal.pone.0041654. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22911836 Free PMC article.
-
An encapsulation of iron homeostasis and virulence in Cryptococcus neoformans.Trends Microbiol. 2013 Sep;21(9):457-65. doi: 10.1016/j.tim.2013.05.007. Epub 2013 Jun 25. Trends Microbiol. 2013. PMID: 23810126 Free PMC article. Review.
-
Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule.PLoS Pathog. 2010 Feb 19;6(2):e1000776. doi: 10.1371/journal.ppat.1000776. PLoS Pathog. 2010. PMID: 20174553 Free PMC article.
References
-
- Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Natural resistance, iron and infection: A challenge for clinical medicine. J Med Microbiol. 2006;55:251–258. - PubMed
-
- Weinberg ED. Iron and susceptibility to infectious disease. Science. 1974;184:952–956. - PubMed
-
- Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: A multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–286. - PubMed
-
- Wandersman C, Delepelaire P. Bacterial iron sources: From siderophores to hemophores. Annu Rev Microbiol. 2004;58:611–647. - PubMed
-
- Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science. 2004;305:1626–1628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

