Discovering mechanisms of signaling-mediated cysteine oxidation
- PMID: 18282483
- PMCID: PMC2408887
- DOI: 10.1016/j.cbpa.2008.01.021
Discovering mechanisms of signaling-mediated cysteine oxidation
Abstract
Accumulating evidence reveals hydrogen peroxide as a key player both as a damaging agent and, from emerging evidence over the past decade, as a second messenger in intracellular signaling. This rather mild oxidant acts upon downstream targets within signaling cascades to modulate the activity of a host of enzymes (e.g. phosphatases and kinases) and transcriptional regulators through chemoselective oxidation of cysteine residues. With the recent development of specific detection reagents for hydrogen peroxide and new chemical tools to detect the generation of the initial oxidation product, sulfenic acid, on reactive cysteines within target proteins, the scene is set to gain a better understanding of the mechanisms through which hydrogen peroxide acts as a second messenger in cell signaling.
Figures
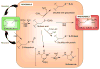
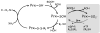

References
-
- Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. Trafficking of both activated IL-1 receptor complexes and activated Nox complexes to endosomal compartments is demonstrated, along with evidence supporting the generation of superoxide and hydrogen peroxide within the lumen of the endosomes. - PMC - PubMed
-
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. - PubMed
-
- Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. - PubMed
-
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. - PubMed
-
- Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

