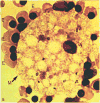
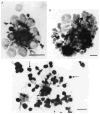
The erythroblastic island
- PMID: 18282516
- PMCID: PMC3234703
- DOI: 10.1016/S0070-2153(07)00002-6
The erythroblastic island
Abstract
Erythroblastic islands are specialized microenvironmental compartments within which definitive mammalian erythroblasts proliferate and differentiate. These islands consist of a central macrophage that extends cytoplasmic protrusions to a ring of surrounding erythroblasts. The interaction of cells within the erythroblastic island is essential for both early and late stages of erythroid maturation. It has been proposed that early in erythroid maturation the macrophages provide nutrients, proliferative and survival signals to the erythroblasts, and phagocytose extruded erythroblast nuclei at the conclusion of erythroid maturation. There is also accumulating evidence for the role of macrophages in promoting enucleation itself. The central macrophages are identified by their unique immunophenotypic signature. Their pronounced adhesive properties, ability for avid endocytosis, lack of respiratory bursts, and consequent release of toxic oxidative species, make them perfectly adapted to function as nurse cells. Both macrophages and erythroblasts display adhesive interactions that maintain island integrity, and elucidating these details is an area of intense interest and investigation. Such interactions enable regulatory feedback within islands via cross talk between cells and also trigger intracellular signaling pathways that regulate gene expression. An additional control mechanism for cellular growth within the erythroblastic islands is through the modulation of apoptosis via feedback loops between mature and immature erythroblasts and between macrophages and immature erythroblasts. The focus of this chapter is to outline the mechanisms by which erythroblastic islands aid erythropoiesis, review the historical data surrounding their discovery, and highlight important unanswered questions.
Figures



References
-
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. - PubMed
-
- Allen TD, Dexter TM. Ultrastructural aspects of erythropoietic differentiation in long-term bone marrow culture. Differentiation. 1982;21:86–94. - PubMed
-
- Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2006;69:69–85. - PubMed
-
- Arkin S, Naprstek B, Guarini L, Ferrone S, Lipton JM. Expression of intercellular adhesion molecule-1 (CD54) on hematopoietic progenitors. Blood. 1991;77:948–953. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

