Dynamic behavior of GFP-CLIP-170 reveals fast protein turnover on microtubule plus ends
- PMID: 18283108
- PMCID: PMC2265578
- DOI: 10.1083/jcb.200707203
Dynamic behavior of GFP-CLIP-170 reveals fast protein turnover on microtubule plus ends
Abstract
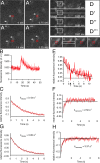
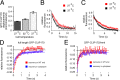
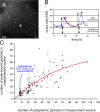
Microtubule (MT) plus end-tracking proteins (+TIPs) specifically recognize the ends of growing MTs. +TIPs are involved in diverse cellular processes such as cell division, cell migration, and cell polarity. Although +TIP tracking is important for these processes, the mechanisms underlying plus end specificity of mammalian +TIPs are not completely understood. Cytoplasmic linker protein 170 (CLIP-170), the prototype +TIP, was proposed to bind to MT ends with high affinity, possibly by copolymerization with tubulin, and to dissociate seconds later. However, using fluorescence-based approaches, we show that two +TIPs, CLIP-170 and end-binding protein 3 (EB3), turn over rapidly on MT ends. Diffusion of CLIP-170 and EB3 appears to be rate limiting for their binding to MT plus ends. We also report that the ends of growing MTs contain a surplus of sites to which CLIP-170 binds with relatively low affinity. We propose that the observed loss of fluorescent +TIPs at plus ends does not reflect the behavior of single molecules but is a result of overall structural changes of the MT end.
Figures

References
-
- Akhmanova, A., and C.C. Hoogenraad. 2005. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr. Opin. Cell Biol. 17:47–54. - PubMed
-
- Akhmanova, A., A.-L. Mausset-Bonnefont, W. Van Cappellen, N. Keijzer, C.C. Hoogenraad, T. Stepanova, K. Drabek, J. van der Wees, M. Mommaas, J. Onderwater, et al. 2005. The microtubule plus end tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis. Genes Dev. 19:2501–2515. - PMC - PubMed
-
- Arnal, I., and R.H. Wade. 1995. How does taxol stabilize microtubules? Curr. Biol. 5:900–908. - PubMed
-
- Arnal, I., C. Heichette, G.S. Diamantopoulos, and D. Chretien. 2004. CLIP-170/tubulin-curved oligomers coassemble at microtubule ends and promote rescues. Curr. Biol. 14:2086–2095. - PubMed
-
- Charpilienne, A., M. Nejmeddine, M. Berois, N. Parez, E. Neumann, E. Hewat, G. Trugnan, and J. Cohen. 2001. Individual rotavirus-like particles containing 120 molecules of fluorescent protein are visible in living cells. J. Biol. Chem. 276:29361–29367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

