Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells
- PMID: 18283112
- PMCID: PMC2265583
- DOI: 10.1083/jcb.200709161
Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells
Abstract
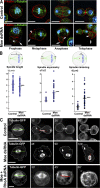
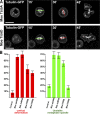
Cell division requires cell shape changes involving the localized reorganization of cortical actin, which must be tightly linked with chromosome segregation operated by the mitotic spindle. How this multistep process is coordinated remains poorly understood. In this study, we show that the actin/membrane linker moesin, the single ERM (ezrin, radixin, and moesin) protein in Drosophila melanogaster, is required to maintain cortical stability during mitosis. Mitosis onset is characterized by a burst of moesin activation mediated by a Slik kinase-dependent phosphorylation. Activated moesin homogenously localizes at the cortex in prometaphase and is progressively restricted at the equator in later stages. Lack of moesin or inhibition of its activation destabilized the cortex throughout mitosis, resulting in severe cortical deformations and abnormal distribution of actomyosin regulators. Inhibiting moesin activation also impaired microtubule organization and precluded stable positioning of the mitotic spindle. We propose that the spatiotemporal control of moesin activation at the mitotic cortex provides localized cues to coordinate cortical contractility and microtubule interactions during cell division.
Figures


References
-
- Bettencourt-Dias, M., R. Giet, R. Sinka, A. Mazumdar, W.G. Lock, F. Balloux, P.J. Zafiropoulos, S. Yamaguchi, S. Winter, R.W. Carthew, et al. 2004. Genome-wide survey of protein kinases required for cell cycle progression. Nature. 432:980–987. - PubMed
-
- Bjorklund, M., M. Taipale, M. Varjosalo, J. Saharinen, J. Lahdenpera, and J. Taipale. 2006. Identification of pathways regulating cell size and cell-cycle progression by RNAi. Nature. 439:1009–1013. - PubMed
-
- Bretscher, A., K. Edwards, and R.G. Fehon. 2002. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3:586–599. - PubMed
-
- Canman, J.C., L.A. Cameron, P.S. Maddox, A. Straight, J.S. Tirnauer, T.J. Mitchison, G. Fang, T.M. Kapoor, and E.D. Salmon. 2003. Determining the position of the cell division plane. Nature. 424:1074–1078. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

