Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization
- PMID: 18283114
- PMCID: PMC2265580
- DOI: 10.1083/jcb.200710074
Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization
Abstract
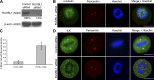
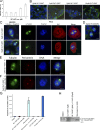
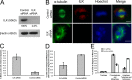
Integrin-linked kinase (ILK) is a serine-threonine kinase and scaffold protein with well defined roles in focal adhesions in integrin-mediated cell adhesion, spreading, migration, and signaling. Using mass spectrometry-based proteomic approaches, we identify centrosomal and mitotic spindle proteins as interactors of ILK. alpha- and beta-tubulin, ch-TOG (XMAP215), and RUVBL1 associate with ILK and colocalize with it to mitotic centrosomes. Inhibition of ILK activity or expression induces profound apoptosis-independent defects in the organization of the mitotic spindle and DNA segregation. ILK fails to localize to the centrosomes of abnormal spindles in RUVBL1-depleted cells. Additionally, depletion of ILK expression or inhibition of its activity inhibits Aurora A-TACC3/ch-TOG interactions, which are essential for spindle pole organization and mitosis. These data demonstrate a critical and unexpected function for ILK in the organization of centrosomal protein complexes during mitotic spindle assembly and DNA segregation.
Figures


Similar articles
-
Beyond focal adhesions: integrin-linked kinase associates with tubulin and regulates mitotic spindle organization.Cell Cycle. 2008 Jul 1;7(13):1899-906. doi: 10.4161/cc.7.13.6204. Epub 2008 Apr 24. Cell Cycle. 2008. PMID: 18604167
-
A critical role of integrin-linked kinase, ch-TOG and TACC3 in centrosome clustering in cancer cells.Oncogene. 2011 Feb 3;30(5):521-34. doi: 10.1038/onc.2010.431. Epub 2010 Sep 13. Oncogene. 2011. PMID: 20838383
-
Mitotic catastrophe and cell death induced by depletion of centrosomal proteins.Cell Death Dis. 2013 Apr 18;4(4):e603. doi: 10.1038/cddis.2013.108. Cell Death Dis. 2013. PMID: 23598415 Free PMC article.
-
The role of TACC3 in mitotic spindle organization.Cytoskeleton (Hoboken). 2017 Oct;74(10):369-378. doi: 10.1002/cm.21388. Epub 2017 Aug 23. Cytoskeleton (Hoboken). 2017. PMID: 28745816 Review.
-
Role of centrosomal adaptor proteins of the TACC family in the regulation of microtubule dynamics during mitotic cell division.Biol Chem. 2013 Nov;394(11):1411-23. doi: 10.1515/hsz-2013-0184. Biol Chem. 2013. PMID: 23787465 Review.
Cited by
-
CP250, a novel acidic coiled-coil protein of the Dictyostelium centrosome, affects growth, chemotaxis, and the nuclear envelope.Mol Biol Cell. 2009 Oct;20(20):4348-61. doi: 10.1091/mbc.e09-03-0180. Epub 2009 Aug 19. Mol Biol Cell. 2009. PMID: 19692569 Free PMC article.
-
Let's huddle to prevent a muddle: centrosome declustering as an attractive anticancer strategy.Cell Death Differ. 2012 Aug;19(8):1255-67. doi: 10.1038/cdd.2012.61. Epub 2012 Jun 1. Cell Death Differ. 2012. PMID: 22653338 Free PMC article. Review.
-
The focal adhesion protein PINCH-1 associates with EPLIN at integrin adhesion sites.J Cell Sci. 2015 Mar 1;128(5):1023-33. doi: 10.1242/jcs.162545. Epub 2015 Jan 20. J Cell Sci. 2015. PMID: 25609703 Free PMC article.
-
Arpc1b, a centrosomal protein, is both an activator and substrate of Aurora A.J Cell Biol. 2010 Jul 12;190(1):101-14. doi: 10.1083/jcb.200908050. Epub 2010 Jul 5. J Cell Biol. 2010. PMID: 20603326 Free PMC article.
-
Keep Calm and Carry on with Extra Centrosomes.Cancers (Basel). 2022 Jan 17;14(2):442. doi: 10.3390/cancers14020442. Cancers (Basel). 2022. PMID: 35053604 Free PMC article. Review.
References
-
- Bendig, G., M. Grimmler, I.G. Huttner, G. Wessels, T. Dahme, S. Just, N. Trano, H.A. Katus, M.C. Fishman, and W. Rottbauer. 2006. Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev. 20:2361–2372. - PMC - PubMed
-
- Bettencourt-Dias, M., R. Giet, R. Sinka, A. Mazumdar, W.G. Lock, F. Balloux, P.J. Zafiropoulos, S. Yamaguchi, S. Winter, R.W. Carthew, et al. 2004. Genome-wide survey of protein kinases required for cell cycle progression. Nature. 432:980–987. - PubMed
-
- Candiano, G., M. Bruschi, L. Musante, L. Santucci, G.M. Ghiggeri, B. Carnemolla, P. Orecchia, L. Zardi, and P.G. Righetti. 2004. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 25:1327–1333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

