The role of fascin in the migration and invasiveness of malignant glioma cells
- PMID: 18283337
- PMCID: PMC2244690
- DOI: 10.1593/neo.07909
The role of fascin in the migration and invasiveness of malignant glioma cells
Abstract
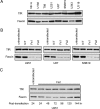
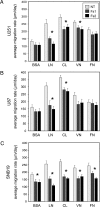
Malignant glioma is the most common primary brain tumor, and its ability to invade the surrounding brain parenchyma is a leading cause of tumor recurrence and treatment failure. Whereas the molecular mechanisms of glioma invasion are incompletely understood, there is growing evidence that cytoskeletal-matrix interactions contribute to this process. Fascin, an actin-bundling protein, induces parallel actin bundles in cell protrusions and increases cell motility in multiple human malignancies. The role of fascin in glioma invasion remains unclear. We demonstrate that fascin is expressed in a panel of human malignant glioma cell lines, and downregulation of fascin expression in glioma cell lines by small interfering RNA (siRNA) is associated with decreased cellular attachment to extracellular matrix (ECM) and reduced migration. Using immunofluorescence analysis, we show that fascin depletion results in a reduced number of filopodia as well as altered glioma cell shape. In vitro invasiveness of U251, U87, and SNB19 glioma cells was inhibited by fascin siRNA treatment by 52.2%, 40.3%, and 23.8% respectively. Finally, we show a decreased invasiveness of U251-GFP cells by fascin knockdown in an ex vivo rat brain slice model system. This is the first study to demonstrate a role for fascin in glioma cell morphology, motility, and invasiveness.
Figures





References
-
- Burger PC, Vogel FS, Green SB, Strike TA. Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer. 1985;56(5):1106–1111. - PubMed
-
- Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25(26):4127–4136. - PubMed
-
- Soffietti R, Leoncini B, Ruda R. New developments in the treatment of malignant gliomas. Expert Rev Neurother. 2007;7(10):1313–1326. - PubMed
-
- Goffman TE, Dachowski LJ, Bobo H, Oldfield EH, Steinberg SM, Cook J, Mitchell JB, Katz D, Smith R, Glatstein E. Long-term followup on National Cancer Institute Phase I/II study of glioblastoma multiforme treated with iododeoxyuridine and hyperfractionated irradiation. J Clin Oncol. 1992;10(2):264–268. - PubMed
-
- Senger D, Cairncross JG, Forsyth PA. Long-term survivors of glioblastoma: statistical aberration or important unrecognized molecular subtype? Cancer J. 2003;9(3):214–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
