Multidrug resistance-associated protein 3 (Mrp3/Abcc3/Moat-D) is expressed in the SAE Squalus acanthias shark embryo-derived cell line
- PMID: 18284333
- PMCID: PMC2597096
- DOI: 10.1089/zeb.2007.0520
Multidrug resistance-associated protein 3 (Mrp3/Abcc3/Moat-D) is expressed in the SAE Squalus acanthias shark embryo-derived cell line
Abstract
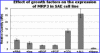
The multidrug resistance-associated protein 3 (MRP3/Mrp3) is a member of the ATP-binding cassette (ABC) protein family of membrane transporters and related proteins that act on a variety of xenobiotic and anionic molecules to transfer these substrates in an ATP-dependent manner. In recent years, useful comparative information regarding evolutionarily conserved structure and transport functions of these proteins has accrued through the use of primitive marine animals such as cartilaginous fish. Until recently, one missing tool in comparative studies with cartilaginous fish was cell culture. We have derived from the embryo of Squalus acanthias, the spiny dogfish shark, the S. acanthias embryo (SAE) mesenchymal stem cell line. This is the first continuously proliferating cell line from a cartilaginous fish. We identified expression of Mrp3 in this cell line, cloned the molecule, and examined molecular and cellular physiological aspects of the protein. Shark Mrp3 is characterized by three membrane-spanning domains and two nucleotide-binding domains. Multiple alignments with other species showed that the shark Mrp3 amino acid sequence was well conserved. The shark sequence was overall 64% identical to human MRP3, 72% identical to chicken Mrp3, and 71% identical to frog and stickleback Mrp3. Highest identity between shark and human amino acid sequence (82%) was seen in the carboxyl-terminal nucleotide-binding domain of the proteins. Cell culture experiments showed that mRNA for the protein was induced as much as 25-fold by peptide growth factors, fetal bovine serum, and lipid nutritional components, with the largest effect mediated by a combination of lipids including unsaturated and saturated fatty acids, cholesterol, and vitamin E.
Figures







References
-
- Annilo T, Chen ZQ, Shulenin S, Constantino J, Thomas L, Lou H, et al. Evolution of the vertebrate ABC gene family: analysis of gene birth and death. Genomics. 2006;88:1–11. - PubMed
-
- Haimeur A, Conseil G, Deeley RG, Cole SP. The MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr Drug Metab. 2004;5:21–53. - PubMed
-
- Soroka CJ, Lee JM, Lee F, Azzaroli A, Boyer JL. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology. 2001;33:783–791. - PubMed
-
- Cai S-Y, Soroka CJ, Ballatori N, Boyer JL. Molecular characterization of a multidrug resistance-associated protein, Mrp2, from the little skate. Am J Physiol Regul Integr Comp Physiol. 2003;284:R125–R130. - PubMed
-
- Lang T, Hitzl M, Burk O, Mornhinweg E, Keil A, Kerb R, et al. Genetic polymorphisms in the multidrug resistance-associated protein 3 (ABCC3, MRP3) gene and relationship to its mRNA and protein expression in human liver. Pharmacogenetics. 2004;14:155–164. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

