Copper accumulation and lipid oxidation precede inflammation and myelin lesions in N,N-diethyldithiocarbamate peripheral myelinopathy
- PMID: 18284930
- PMCID: PMC2429851
- DOI: 10.1016/j.taap.2008.01.005
Copper accumulation and lipid oxidation precede inflammation and myelin lesions in N,N-diethyldithiocarbamate peripheral myelinopathy
Abstract
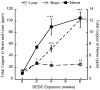
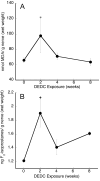
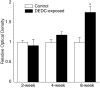
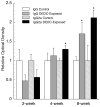
Dithiocarbamates have a wide spectrum of applications in industry, agriculture and medicine with new applications being actively investigated. One adverse effect of dithiocarbamates is the neurotoxicity observed in humans and experimental animals. Results from previous studies have suggested that dithiocarbamates elevate copper and promote lipid oxidation within myelin membranes. In the current study, copper levels, lipid oxidation, protein oxidative damage and markers of inflammation were monitored as a function of N,N-diethyldithiocarbamate (DEDC) exposure duration in an established model for DEDC-mediated myelinopathy in the rat. Intra-abdominal administration of DEDC was performed using osmotic pumps for periods of 2, 4, and 8 weeks. Metals in brain, liver and tibial nerve were measured using ICP-MS and lipid oxidation assessed through HPLC measurement of malondialdehyde in tibial nerve, and GC/MS measurement of F(2) isoprostanes in sciatic nerve. Protein oxidative injury of sciatic nerve proteins was evaluated through quantification of 4-hydroxynonenal protein adducts using immunoassay, and inflammation monitored by quantifying levels of IgGs and activated macrophages using immunoassay and immunohistochemistry methods, respectively. Changes in these parameters were then correlated to the onset of structural lesions, determined by light and electron microscopy, to delineate the temporal relationship of copper accumulation and oxidative stress in peripheral nerve to the onset of myelin lesions. The data provide evidence that DEDC mediates lipid oxidation and elevation of total copper in peripheral nerve well before myelin lesions or activated macrophages are evident. This relationship is consistent with copper-mediated oxidative stress contributing to the myelinopathy.
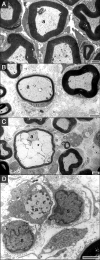
Figures


Similar articles
-
N,N-diethyldithiocarbamate promotes oxidative stress prior to myelin structural changes and increases myelin copper content.Toxicol Appl Pharmacol. 2009 Aug 15;239(1):71-9. doi: 10.1016/j.taap.2009.05.017. Epub 2009 May 23. Toxicol Appl Pharmacol. 2009. PMID: 19467251 Free PMC article.
-
N,N-diethyldithiocarbamate produces copper accumulation, lipid peroxidation, and myelin injury in rat peripheral nerve.Toxicol Sci. 2004 Sep;81(1):160-71. doi: 10.1093/toxsci/kfh190. Epub 2004 Jun 8. Toxicol Sci. 2004. PMID: 15187237
-
Peripheral nerve protein expression and carbonyl content in N,N-diethlydithiocarbamate myelinopathy.Chem Res Toxicol. 2007 Mar;20(3):370-9. doi: 10.1021/tx6003453. Epub 2007 Feb 27. Chem Res Toxicol. 2007. PMID: 17323979 Free PMC article.
-
Nitrogen substituent polarity influences dithiocarbamate-mediated lipid oxidation, nerve copper accumulation, and myelin injury.Chem Res Toxicol. 2009 Jan;22(1):218-26. doi: 10.1021/tx8003714. Chem Res Toxicol. 2009. PMID: 19093748 Free PMC article.
-
Metal-induced hepatotoxicity.Semin Liver Dis. 1996 Feb;16(1):3-12. doi: 10.1055/s-2007-1007214. Semin Liver Dis. 1996. PMID: 8723319 Review.
Cited by
-
Ziram and sodium N,N-dimethyldithiocarbamate inhibit ubiquitin activation through intracellular metal transport and increased oxidative stress in HEK293 cells.Chem Res Toxicol. 2015 Apr 20;28(4):682-90. doi: 10.1021/tx500450x. Epub 2015 Mar 23. Chem Res Toxicol. 2015. PMID: 25714994 Free PMC article.
-
Potentiation of antibiofilm activity of amphotericin B by superoxide dismutase inhibition.Oxid Med Cell Longev. 2013;2013:704654. doi: 10.1155/2013/704654. Epub 2013 Sep 1. Oxid Med Cell Longev. 2013. PMID: 24078861 Free PMC article.
-
N,N-diethyldithiocarbamate promotes oxidative stress prior to myelin structural changes and increases myelin copper content.Toxicol Appl Pharmacol. 2009 Aug 15;239(1):71-9. doi: 10.1016/j.taap.2009.05.017. Epub 2009 May 23. Toxicol Appl Pharmacol. 2009. PMID: 19467251 Free PMC article.
-
Measurement of isoprostanes as markers of oxidative stress in neuronal tissue.Curr Protoc Toxicol. 2009 Feb;Chapter 12(Supplement 39):Unit12.14. doi: 10.1002/0471140856.tx1214s39. Curr Protoc Toxicol. 2009. PMID: 20191108 Free PMC article. Review.
-
Peripheral nerve and brain differ in their capacity to resolve N,N-diethyldithiocarbamate-mediated elevations in copper and oxidative injury.Toxicology. 2010 Jul-Aug;274(1-3):10-7. doi: 10.1016/j.tox.2010.04.018. Epub 2010 May 7. Toxicology. 2010. PMID: 20452388 Free PMC article.
References
-
- Bach SP, Chinery R, O’Dwyer ST, Potten CS, Coffey RJ, Watson AJ. Pyrrolidinedithiocarbamate increases the therapeutic index of 5-fluorouracil in a mouse model. Gastroenterology. 2000;118:81–89. - PubMed
-
- Calviello G, Filippi GM, Toesca A, Palozza P, Maggiano N, Nicuolo FD, Serini S, Azzena GB, Galeotti T. Repeated exposure to pyrrolidine-dithiocarbamate induces peripheral nerve alterations in rats. Toxicol Lett. 2005;158:61–71. - PubMed
-
- Cisternas FA, Tapia G, Arredondo M, Cartier-Ugarte D, Romanque P, Sierralta WD, Vial MT, Videla LA, Araya M. Early histological and functional effects of chronic copper exposure in rat liver. Biometals. 2005;18:541–551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

