Anaplasma phagocytophilum MSP2(P44)-18 predominates and is modified into multiple isoforms in human myeloid cells
- PMID: 18285495
- PMCID: PMC2346672
- DOI: 10.1128/IAI.01594-07
Anaplasma phagocytophilum MSP2(P44)-18 predominates and is modified into multiple isoforms in human myeloid cells
Abstract
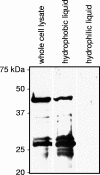
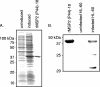
Anaplasma phagocytophilum is the etiologic agent of human granulocytic anaplasmosis. MSP2(P44), the bacterium's major surface protein, is encoded by a paralogous gene family and has been implicated in a variety of pathobiological processes, including antigenic variation, host adaptation, adhesion, porin activity, and structural integrity. The consensus among several studies performed at the DNA and RNA levels is that a heterogeneous mix of a limited number of msp2(p44) transcripts is expressed by A. phagocytophilum during in vitro cultivation. Such analyses have yet to be extended to the protein level. In this study, we used proteomic and molecular approaches to determine that MSP2(P44)-18 is the predominant if not the only paralog expressed and is modified into multiple 42- to 44-kDa isoforms by A. phagocytophilum strain HGE1 during infection of HL-60 cells. The msp2(p44) expression profile was homogeneous for msp2(p44)-18. Thus, MSP2(P44)-18 may have a fitness advantage in HL-60 cell culture in the absence of selective immune pressure. Several novel 22- to 27-kDa MSP2 isoforms lacking most of the N-terminal conserved region were also identified. A. phagocytophilum MSP2(P44) orthologs expressed by other pathogens in the family Anaplasmataceae are glycosylated. Gas chromatography revealed that recombinant MSP2(P44)-18 is modified by glucose, galactose, xylose, mannose, and trace amounts of other glycosyl residues. These data are the first to confirm differential modification of any A. phagocytophilum MSP2(P44) paralog and the first to provide evidence for expression of truncated versions of such proteins.
Figures



Similar articles
-
Differential expression and glycosylation of anaplasma phagocytophilum major surface protein 2 paralogs during cultivation in sialyl Lewis x-deficient host cells.Infect Immun. 2009 May;77(5):1746-56. doi: 10.1128/IAI.01530-08. Epub 2009 Feb 17. Infect Immun. 2009. PMID: 19223475 Free PMC article.
-
Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum.Infect Immun. 2003 Apr;71(4):1706-18. doi: 10.1128/IAI.71.4.1706-1718.2003. Infect Immun. 2003. PMID: 12654783 Free PMC article.
-
Anaplasma phagocytophilum has a functional msp2 gene that is distinct from p44.Infect Immun. 2004 Jul;72(7):3883-9. doi: 10.1128/IAI.72.7.3883-3889.2004. Infect Immun. 2004. PMID: 15213131 Free PMC article.
-
Molecular Detection and Characterization of p44/msp2 Multigene Family of Anaplasma phagocytophilum from Haemaphysalis longicornis in Mie Prefecture, Japan.Jpn J Infect Dis. 2019 May 23;72(3):199-202. doi: 10.7883/yoken.JJID.2018.485. Epub 2019 Jan 31. Jpn J Infect Dis. 2019. PMID: 30700658
-
Persistent Infections and Immunity in Ruminants to Arthropod-Borne Bacteria in the Family Anaplasmataceae.Annu Rev Anim Biosci. 2016;4:177-97. doi: 10.1146/annurev-animal-022513-114206. Epub 2015 Dec 23. Annu Rev Anim Biosci. 2016. PMID: 26734888 Review.
Cited by
-
Molecular characterization of Ehrlichia interactions with tick cells and macrophages.Front Biosci (Landmark Ed). 2009 Jan 1;14(9):3259-73. doi: 10.2741/3449. Front Biosci (Landmark Ed). 2009. PMID: 19273271 Free PMC article. Review.
-
Fucosylation enhances colonization of ticks by Anaplasma phagocytophilum.Cell Microbiol. 2010 Sep 1;12(9):1222-34. doi: 10.1111/j.1462-5822.2010.01464.x. Epub 2010 Mar 19. Cell Microbiol. 2010. PMID: 20331643 Free PMC article.
-
Anaplasma phagocytophilum Asp14 is an invasin that interacts with mammalian host cells via its C terminus to facilitate infection.Infect Immun. 2013 Jan;81(1):65-79. doi: 10.1128/IAI.00932-12. Epub 2012 Oct 15. Infect Immun. 2013. PMID: 23071137 Free PMC article.
-
Variant-specific and diminishing immune responses towards the highly variable MSP2(P44) outer membrane protein of Anaplasma phagocytophilum during persistent infection in lambs.Vet Immunol Immunopathol. 2010 Feb 15;133(2-4):117-24. doi: 10.1016/j.vetimm.2009.07.009. Epub 2009 Jul 30. Vet Immunol Immunopathol. 2010. PMID: 19695712 Free PMC article.
-
Mass spectrometric analysis of Ehrlichia chaffeensis tandem repeat proteins reveals evidence of phosphorylation and absence of glycosylation.PLoS One. 2010 Mar 4;5(3):e9552. doi: 10.1371/journal.pone.0009552. PLoS One. 2010. PMID: 20209062 Free PMC article.
References
-
- Barbet, A. F., J. T. Agnes, A. L. Moreland, A. M. Lundgren, A. R. Alleman, S. M. Noh, K. A. Brayton, U. G. Munderloh, and G. H. Palmer. 2005. Identification of functional promoters in the msp2 expression loci of Anaplasma marginale and Anaplasma phagocytophilum. Gene 35389-97. - PubMed
-
- Barbet, A. F., P. F. Meeus, M. Belanger, M. V. Bowie, J. Yi, A. M. Lundgren, A. R. Alleman, S. J. Wong, F. K. Chu, U. G. Munderloh, and S. D. Jauron. 2003. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect. Immun. 711706-1718. - PMC - PubMed
-
- Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 2561604-1607. - PubMed
-
- Carlyon, J. A., W. T. Chan, J. Galan, D. Roos, and E. Fikrig. 2002. Repression of rac2 mRNA expression by Anaplasma phagocytophila is essential to the inhibition of superoxide production and bacterial proliferation. J. Immunol. 1697009-7018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

