Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1
- PMID: 18285569
- PMCID: PMC3071982
- DOI: 10.1161/CIRCULATIONAHA.107.710111
Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1
Abstract
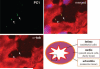
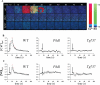
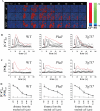
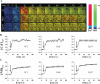
Background: When challenged with extracellular fluid shear stress, vascular endothelial cells are known to release nitric oxide, an important vasodilator. Here, we show that the ability of cultured endothelial cells to sense a low range of fluid shear depends on apical membrane organelles, called cilia, and that cilia are compartments required for proper localization and function of the mechanosensitive polycystin-1 molecule.
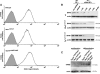
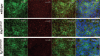
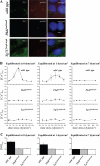
Methods and results: Cells with the Pkd1(null/null) or Tg737(orpk/orpk) mutation encoded for polycystin-1 or polaris, respectively, are unable to transmit extracellular shear stress into intracellular calcium signaling and biochemical nitric oxide synthesis. Cytosolic calcium and nitric oxide recordings further show that fluid shear sensing is a cilia-specific mechanism because other mechanical or pharmacological stimulation does not abolish calcium and nitric oxide signaling in polycystin-1 and polaris mutant endothelial cells. Polycystin-1 localized in the basal body of Tg737(orpk/orpk) endothelial cells is insufficient for a fluid shear stress response. Furthermore, the optimal shear stress to which the cells respond best does not alter the apical cilia structure but modifies the responsiveness of cells to higher shear stresses through proteolytic modification of polycystin-1.
Conclusions: We demonstrate for the first time that polycystin-1 (required for cilia function) and polaris (required for cilia structure) are crucial mechanosensitive molecules in endothelial cells. We propose that a distinctive communication with the extracellular microenvironment depends on the proper localization and function of polycystin-1 in cilia.
Figures

Comment in
-
Deciphering the endothelial shear stress sensor.Circulation. 2008 Mar 4;117(9):1124-6. doi: 10.1161/CIRCULATIONAHA.107.753889. Circulation. 2008. PMID: 18316496 No abstract available.
References
-
- Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol. 2002;282:F541–F552. - PubMed
-
- Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science. 1994;264:1329–1333. - PubMed
-
- Polycystic kidney disease: the complete structure of the PKD1 gene and its protein: the International Polycystic Kidney Disease Consortium. Cell. 1995;81:289–298. - PubMed
-
- Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26:844–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL064867/HL/NHLBI NIH HHS/United States
- P30 DK074038/DK/NIDDK NIH HHS/United States
- R01 DK051050/DK/NIDDK NIH HHS/United States
- R01 DK080640/DK/NIDDK NIH HHS/United States
- HL084451/HL/NHLBI NIH HHS/United States
- R01 DK040703/DK/NIDDK NIH HHS/United States
- R21 HL084451/HL/NHLBI NIH HHS/United States
- P01 CA045548/CA/NCI NIH HHS/United States
- R37 DK051050/DK/NIDDK NIH HHS/United States
- CA45548/CA/NCI NIH HHS/United States
- DK40703/DK/NIDDK NIH HHS/United States
- DK51050/DK/NIDDK NIH HHS/United States
- HL64867/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

